Fox News Flash top headlines for October 20
Fox News Flash top headlines are here. Check out what's clicking on Foxnews.com.
The White House on Wednesday unveiled a vaccination rollout plan for children ages 5 to 11 ahead of a possible authorization to administer COVID shots to children by the Food and Drug Administration and the Centers for Disease Control and Prevention.
The start of a COVID vaccination program for children ages 5-11 is dependent on the "independent FDA and CDC process and timeline," which is set for later this month, and early November, the White House said. The administration rolled out the plan so it "will be ready to begin getting shots in arms in the days following a final CDC recommendation."
PFIZER-BIONTECH SUBMIT COVID-19 VACCINE DATA TO FDA FOR KIDS 5-11
Pfizer and BioNTech requested FDA emergency authorization of their COVID-19 vaccine for children ages 5 to 11 earlier this month.
The Biden administration's plan is set to mobilize a "comprehensive effort" across the public and private sectors to ensure the supply, sites and support are available to get children "vaccinated and protected against the virus."
The administration said it has procured enough vaccine to support vaccinating the 28 million children in the U.S. between the ages of 5 and 11 years old.
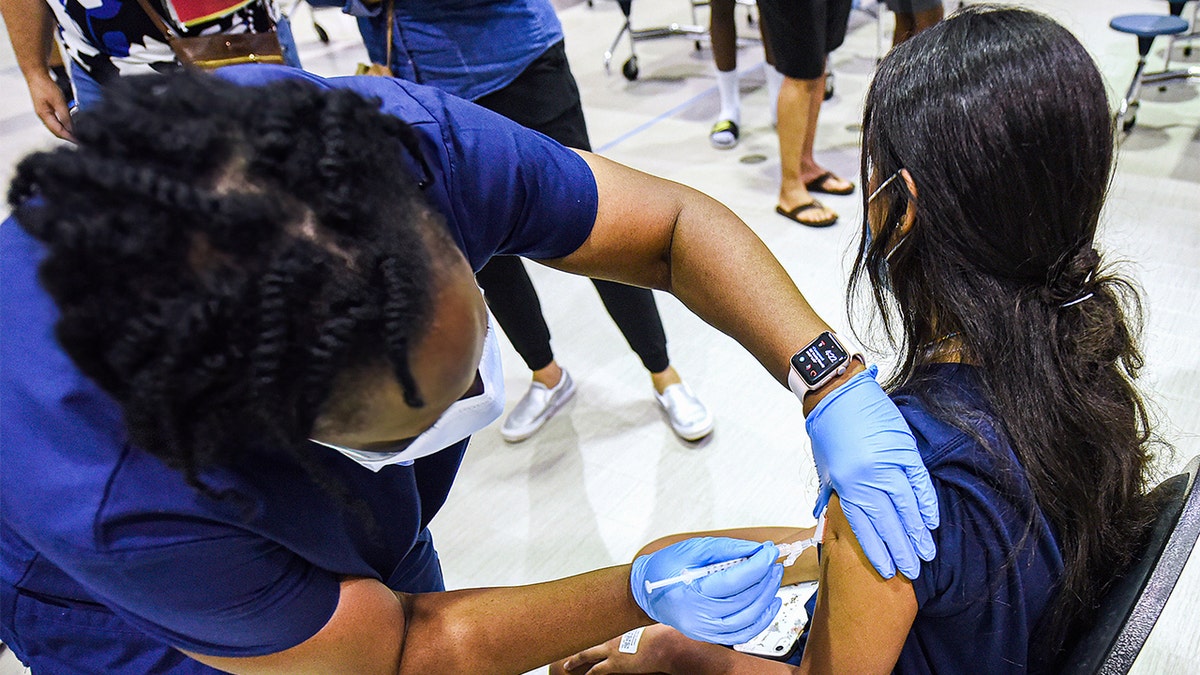
A nurse gives a girl a dose of the Pfizer vaccine at a COVID-19 vaccine clinic at Lyman High School in Longwood on the day before classes begin for the 2021-22 school year. (Photo by Paul Hennessy/SOPA Images/LightRocket via Getty Images) (Paul Hennessy/SOPA Images/LightRocket via Getty Images)
"If authorized by the FDA and recommended by the CDC, the Pfizer-BioNTech vaccine for 5-11 year olds will be a dose and formula specifically for this age group," the White House said. "The vaccine will have packaging available in smaller configurations that will make it easier for physicians’ offices and other smaller, community-based providers to offer the vaccine to kids and their families."
Pending the FDA's authorization, the packaging configuration would be 10-dose vials in cartons of 10 vials each (100 doses total) delivered in a newly updated product shipper. Officials said the vaccine can then be stored for up to 10 weeks at standard refrigeration temperature, and six months at ultracold temperatures.
The administration also plans to make vaccination "accessible and conveniently located" to families across the country, by creating vaccination clinics at doctors' offices, hospitals, pharmacies, community health centers, and school-and community-based sites. Officials said more than 25,000 pediatric and primary care provider sites will provide vaccines for children, as well as tens of thousands of other provider locations.
Meanwhile, to ensure parents "have the information they need to make informed choices for their families," the Department of Health and Human Services is set to conduct a national public education campaign to reach parents and guardians with "accurate and culturally-responsive information about the vaccine and the risks that COVID-19 poses to children."
PFIZER, BIONTECH REQUEST COVID-19 VACCINE EMERGENCY AUTHORIZATION FOR KIDS AGES 5 TO 11
The campaign is set to "invest heavily" in "trusted messengers; work with schools, state and local health departments, faith leaders, and national and community organizations to increase vaccine confidence; create forums for parents to ask questions to pediatricians; and reach out to parents directly through press and social media across channels and in multiple languages."
"Central to this work will be close collaboration with the major national organizations, and their local affiliates, that reach families across the country – including provider and parent organizations and other key partners from across the public and private sectors," officials said.
Meanwhile, for those families that do not have a consistent medical provider for their children, the surgeon general is set to enlist pediatricians and community leaders to talk to Americans "directly" via social media channels and through visits to hard-hit and high-risk communities.
As for whether public schools across the country will begin to require COVID vaccinations for children, administration officials said school vaccination requirements "have been around for decades," but that those decisions will be made at the state and local levels.
The potential plan for kid ages 5-11 comes after millions of adolescents between the ages of 12 and 17 have been "safely vaccinated," the administration said.
"We know vaccines work," the White House said. "Fully vaccinated individuals are 10 times less likely to be hospitalized with COVID-19 and have a high degree of protection, including against the delta variant."
They added: "The consequences of a pediatric COVID-19 case can be serious and potentially last months."
The administration also maintained that, as part of the American Rescue Plan, the federal government will continue to give funds and "unprecedented levels of federal support" to states, including full reimbursement for vaccination operations and outreach programs.
CLICK HERE TO GET THE FOX NEWS APP
Meanwhile, Pfizer's submission for EUA at the FDA comes after the company submitted clinical trial data from a COVID-19 vaccine study among kids ages 5-11 to the FDA. The trial data included recent findings among 2,268 participants ages 5-11, which suggested a smaller dose shot was safe, well tolerated and resulted in neutralizing antibody responses. The companies selected a two-dose regimen of 10 microgram (ug) doses for kids ages 5-11, versus the two-dose regimen of 30 ug doses used for everyone 12 and older.
The companies previously announced that trial results on younger age groups, like children ages 6 months to under 5 years, are expected by the fourth quarter.
The FDA has scheduled an advisory committee meeting on Oct. 26 to inform its decision-making on the Pfizer-BioNTech vaccine for kids ages 5-11.














































