NY clinics 'inappropriately' handing out vaccines should be under federal investigation: Dr. Marc Siegel
New York clinics face criminal probe over vaccines; reaction from Fox News medical contributor Dr. Marc Siegel.
For one New York-based single mother of young children, who suffers from respiratory problems, Christmas came early when she learned through word-of-mouth that – despite conflicting information as to who was eligible to receive the coronavirus vaccine – her underlying condition meant she could indeed be inoculated at a Brooklyn-based health clinic.
"I was so relieved and grateful," the mother and working professional, who requested anonymity for privacy reasons, told Fox News. "And shocked I could get it already."
A week ago, she filled out a brief form online and was later informed that she had an appointment at a ParCare clinic in Brooklyn for Tuesday, the very same week that Moderna was shipping its highly anticipated vaccines across the country.
"It all seemed normal, no different than going to any other urgent care and everything was by the book," the woman, who got the jab almost a week ago, continued, explaining that the clinic took some medical insurances for the shot, but not hers, charging her $150 out of pocket. "But the doctor didn't ask me which box I checked on form or why I qualified for the vaccine."
COVID-19 VACCINE ROLLOUT PLAGUED BY PROTESTS, FAILED TECHNOLOGY AND FRONT-LINE WORKERS MISSING OUT
Word had spread in the days leading up to the Moderna rollout that the prominent ParCare clinic was authorized to administer the shot.
"The vaccines will be made available on a first-come, first-served basis," the company's ad read. "We have set up a special system where you can reserve your slot."
The advertisement urged New Yorkers to go to parcarevaccine.com to submit information or scan the barcode featured on its posters.
The poster said the vaccine was for "high risk, the elderly, and those with underlying conditions," which New York state argues is not its decree at present.
The woman said a strange thing happened Friday when she received a seemingly automated email – several days after the shot – thanking her for submitting her form online and cautioning patients not to show up unless they were issued an appointment, although her date had since passed.
A day later, the New York Health Department (DOH) Commissioner Howard Zucker announced ParCare Community Health Network – with five locations in Brooklyn and one in Manhattan – was under criminal investigation over concerns it "may have fraudulently obtained" the coveted COVID-19 vaccine" and "transferred it to facilities in other parts of the state in violation of state guidelines and diverted it to members of the public," contrary to the state's plan to administer it first to front-line health care workers and first responders, as well as nursing home residents and staffers.
"We take this very seriously, and DOH will be assisting State Police in a criminal investigation into this matter," the statement concluded. "Anyone found to have knowingly participated in this scheme will be held accountable to the fullest extent of the law."
US COVID-19 VACCINATIONS LIKELY TO FALL SHORT OF 20M END-OF-YEAR GOAL
However, a spokesperson for ParCare told Fox News on Monday that the clinic was given contrary information by a DOH representative on Dec. 21, the day it received the Moderna shipment.
"There was nothing illegal here, no black markets transfer. The company was told – over the phone – it was allowed to re-distribute the doses to other clinics in its network," the rep said.
A ParCare spokesperson also affirmed on Monday it has since "proactively returned" its existing inventory of vaccines to the state "pending the department's review," leaving those who did receive it perplexed as to whether they will reap the full benefits and receive the follow-up jab. As it is formulated, the Moderna vaccine requires a booster shot 28 days after the first one.
The spokesperson also said it has provided the documentation regarding the proper receipt of the vaccines to the NYS DOH and has vehemently denied any wrongdoing in its bid to obtain vaccine doses.
"Our record of working hand-in-hand with the city and state of New York is second-to-none. ParCare followed all NYS DOH procedures for obtaining the Moderna vaccine and was approved by NYS DOH for distribution and by CDC as a network site," the representative said. "We are confident the end result of that review will show that ParCare at all times exerted best efforts to comply with all NYS DOH requirements and will allow us to continue to achieve our number one goal of providing these critical vaccines to the New Yorkers who need them most."
A copy of a packing slip given to Fox News clarifies that the vaccines were shipped directly to ParCare in Monroe, N.Y., from a McKesson pharmaceutical supply warehouse in Shepherdsville, Ky., according to the paper.
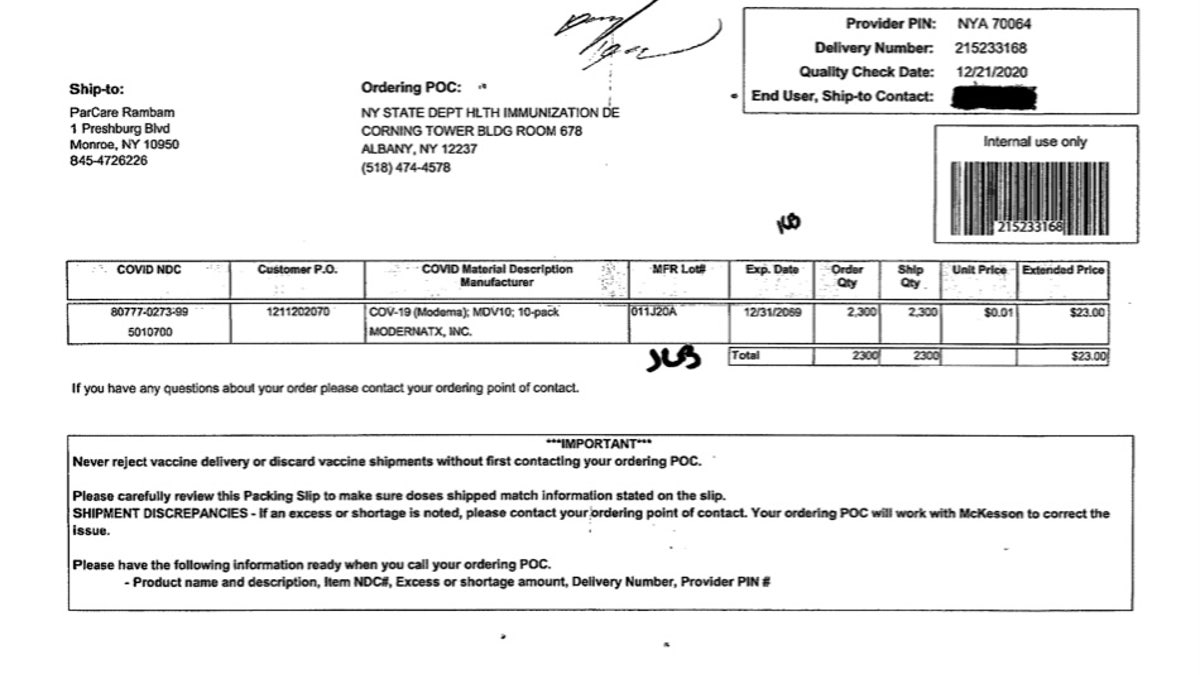
A copy of a packing slip given to Fox News clarifies that the vaccines were shipped directly to ParCare in Monroe, N.Y., from a McKesson pharmaceutical-supply warehouse in Shepherdsville, Ky., according to the paper, driving forth the company's claim that they were not illegally obtained as the state has speculated. (ParCare spokesperson)
Soon after the state announced the criminal probe, ParCare released a statement insisting it has "striven to provide critical healthcare services and administer COVID-19 vaccinations to those qualified to receive them under the New York State Department of Health's guidelines." The company also underscored that it "has a long history of partnering with the City of New York to provide vital healthcare services to New Yorkers who need them most."
However, what many experts find confusing is that despite the mandate that front-line health care workers and those in nursing homes and aged-care facilities that have weathered more than half the deaths in the state be the first to receive the vaccine, ParCare candidly advertised via social media ads and a big campaign that it was receiving a shipment of some 2,300 does from Moderna.
ParCare is said to have administered more than 850 initial shots to date. It remains unclear how many of those were given to individuals who qualify under the state's current guidelines.
While the issue is not clear, ParCare has stated that it will "do everything in (its) power to make sure that the state understands that our patients are our priority and that everyone receives their second dose accordingly."
"One person receiving the full vaccination dose could mean at least one more fatality averted," noted Kagya Amoako, an associate professor of biomedical engineering at the University of New Haven. "So it is highly recommended that the administration of their second doses be considered. However, it may be wise for the state to perform additional reviews of the medical records to make sure a second dose will not lead to adverse events."
CORONAVIRUS VACCINE DISTRIBUTION PLANS: STATE-BY-STATE BREAKDOWN
According to some legal experts, it may all amount to a mammoth misunderstanding.
"I doubt that ParCare Medical Center CEO would have advertised him receiving the COVID vaccination if he understood it was illegal," Amoako explained. "Stating that they administer vaccinations to those qualified to receive them under the state's DOH guideline, which includes front-line health care workers and first responders, is not the same as stating that the recommended sequence of vaccination by the group was adhered to.
"Teeth may need to be added the vaccination guideline language to clear up any confusion about consequences or lack thereof of not following the guidelines."
CLICK HERE TO GET THE FOX NEWS APP
Yet others contend that New York state, amid the throes of another uptick in cases, may push the case in other directions.
"I don't believe that providing the vaccine itself to people who are not in the designated category is in itself a crime unless ParCare was being paid to allow some to 'skip the line' despite the guidelines or they lied to get the vaccine in the first place," added criminal defense attorney Troy Slaten. "Potential crimes could be in procurement under false pretenses, which is possibly criminal fraud. And if more than one person is a part of the scheme, then there is a conspiracy to commit fraud as well."
Thus, if ParCare obtained the vaccine promising to provide it only per the government guidelines and then took money to provide it to others who are not yet eligible due to the current scarcity of supply, then that would be fraud, he said.
"Each fraudulent incident (vaccination) could be charged as a separate felony," Slaten surmised.
The New York governor's office and biotech firm Moderna did not respond to a request for further comments on the matter.










































