Celine Dion diagnosed with stiff person syndrome
Neurologist Dr. Scott Newsome, director of the Johns Hopkins Stiff Person Syndrome Center, explains the popular singer's diagnosis.
Patients with stiff person syndrome are one step closer to having access to a new treatment.
Kyverna Therapeutics’ new drug, KYV-101, has been designated by the U.S. Food and Drug Administration (FDA) a Regenerative Medicine Advanced Therapy (RMAT), the company announced on Monday.
A drug is eligible for RMAT designation if it is "intended to treat, modify, reverse or cure a serious or life-threatening disease or condition" and if "preliminary clinical evidence indicates that the drug has the potential to address unmet medical needs for such disease or condition," according to the FDA’s website.
One of the benefits of RMAT is that Kyverna will be able to work closely with the FDA to help support accelerated development, review and approval, according to the company.
The FDA’s decision was based on positive results from clinical trials with patients, a company press release stated.

Carrie Robinette, left, was diagnosed with stiff person syndrome in 2023. The FDA named KYV-101 a Regenerative Medicine Advanced Therapy (RMAT) on Monday. (Carrie Robinette; iStock)
A rare neurological disorder, stiff person syndrome affects only one or two people for every million — including singer Celine Dion.
The disease can have a devastating impact, causing muscle rigidity, pain, spasms and loss of mobility.
LIKE CELINE DION, PENNSYLVANIA MAN IS FIGHTING STIFF PERSON SYNDROME WITH ‘EVERYTHING I HAVE’
Kyverna Therapeutics, based in Emeryville, California, developed the new CAR-T cell therapy, KYV-101, with the goal of "resetting" the immune systems of patients with autoimmune diseases, according to the company.
"As a physician dedicated to optimizing diagnosis and treatments for patients with autoimmune neurological disorders, I am grateful to be able to witness and contribute to the advancement of treatments and patient outcomes in stiff person syndrome via collaborative research efforts with leading players in the field and the support of the FDA oversight," Amanda Piquet, M.D., director of the Autoimmune Neurology Program at CU Anschutz Medical Campus in Aurora, Colorado, told Fox News Digital via email.

Céline Dion shared her stiff person syndrome diagnosis with the world in Dec. 2022. (Edward Berthelot/GC Images/Getty Images)
Piquet was not involved in the development of KYV-101, but did take part in one of the medical advisory boards.
Peter Maag, PhD, CEO of Kyverna, also commented on the announcement.
"This RMAT designation means science agrees that fast-tracking this treatment could save lives."
"Kyverna is focused on supporting patients and their clinical unmet needs, building patient-centric clinical trials to allow access to the latest medical advances in the CAR-T space for autoimmune disease," Maag told Fox News Digital in a written statement.
"We are proud to be able to investigate the potential of safe and effective long-lasting treatment that can lead to meaningful, durable remission for patients suffering from autoimmune diseases like SPS."
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
Patients also reacted to the news with a sense of excitement.
"From the moment I learned what Kyverna’s treatment had done for the SPS patient in Germany, my dream was that it would get FDA-approved quickly so we could all benefit," Carrie Robinette, 45, from San Diego, California, told Fox News Digital.

A drug is eligible for RMAT designation if it is "intended to treat, modify, reverse or cure a serious or life-threatening disease or condition" and if "preliminary clinical evidence indicates that the drug has the potential to address unmet medical needs for such disease or condition," according to the FDA’s website. (REUTERS/Andrew Kelly/File photo)
Robinette, a Navy wife and mother, was diagnosed with stiff person syndrome in spring 2023.
"This RMAT designation means science agrees that fast-tracking this treatment could save lives," she added. "I’m thrilled for the entire autoimmune disease community."
CLICK HERE TO GET THE FOX NEWS APP
During clinical trials, 50 patients with oncological and autoimmune conditions have been treated with KYV-101 in more than 15 locations in Europe and the U.S., according to the company.
Kyverna will now continue collecting data from the drug’s Phase 2 trials for stiff person syndrome, multiple sclerosis and myasthenia gravis.
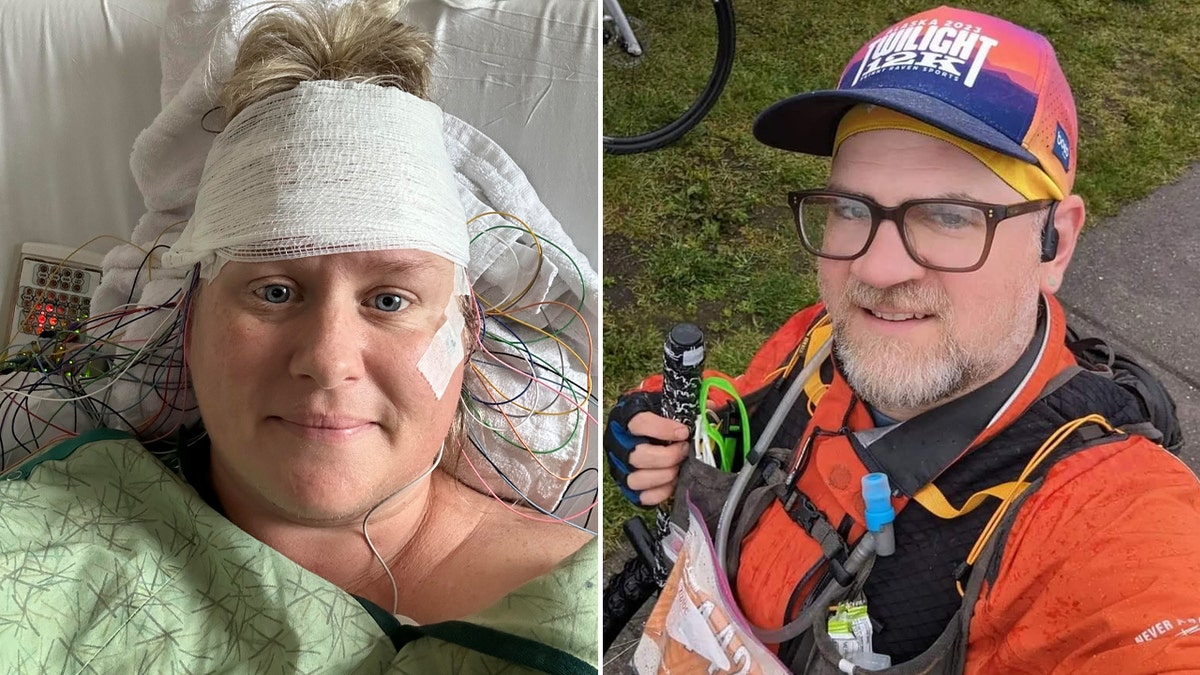
Carrie Robinette, 45, from San Diego, California, at left, and Corwyn Wilkey, 44, who lives in Anchorage, Alaska, right, are both living with stiff person syndrome. (Carrie Robinette; Corwyn Wilkey)
Phase 1 and 2 trials are also underway for systemic sclerosis and lupus.
"We are eager to begin generating data from our sponsored trial to advance the knowledge on a potential immunological reset of the patient’s immune system," Maag said in the company’s release.
For more Health articles, visit www.foxnews/health
Added Piquet, "Stiff-person syndrome has devastating and life-altering effects on patients suffering from this rare autoimmune disease … I look forward to the data that will emerge from the KYSA-8 trial, as this trial could drastically change the treatment landscape for SPS."
Fox News Digital reached out to the FDA for additional comment.