CDC pushes back plans to release post-vaccination guidelines
Fox News medical contributor Dr. Janette Nesheiwat says the CDC will still recommend social distancing for Americans until more Americans are vaccinated and the U.S. is closer to herd immunity.
Some receipts of Moderna’s COVID-19 vaccine have developed a delayed skin reaction to the jab days after receiving it, several doctors wrote in a letter published in the New England Journal of Medicine this week.
Doctors in the letter detailed 12 cases of delayed skin reactions that appeared some four to 11 days following the first dose of the Moderna jab, with an average of eight days. About half of the patients also developed a skin reaction following the second dose, though it was less severe.
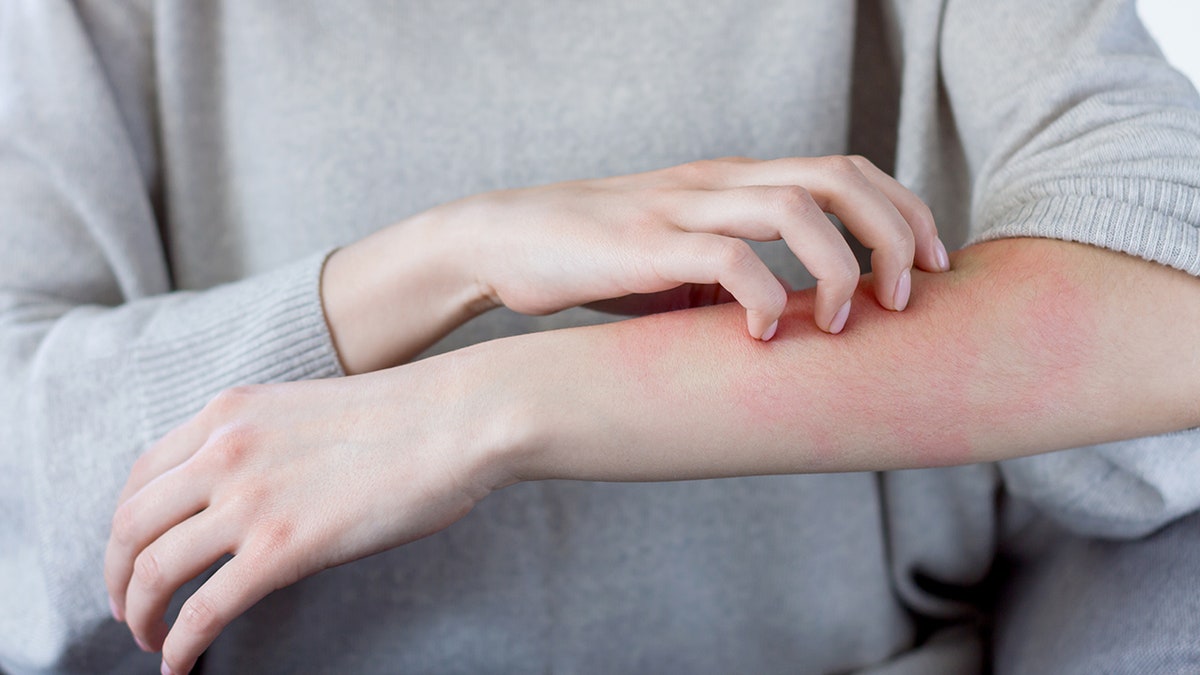
The doctors noted that all vaccine recipients went on to receive the second dose. (iStock)
Most were treated with antihistamines and ice, but some patients required steroid treatments that were prescribed either in a topical or pill form. Most skin rashes resolved after four to five days.
The rashes were harmless but could be confused for an infection, which resulted in the unnecessary use of antibiotics in at least one patient they developed this reaction, they wrote in the letter.
CDC DELAYS GUIDANCE FOR COVID-19 VACCINATED POPULATION
"Clinicians may not be prepared to address delayed local reactions to the mRNA-1273 vaccine. Given the scale-up of mass vaccination campaigns across the world, these reactions are likely to generate concerns among patients and requests for evaluation. These reactions have not been consistently recognized, guidance regarding the second dose of vaccine has varied, and many patients have unnecessarily received antibiotic agents," they wrote. "We hope this letter encourages additional reporting and communication regarding the epidemiologic characteristics, causes, and implications of these delayed cutaneous reactions, since this information might allay the concerns of patients, encourage completion of vaccination, and minimize the unnecessary use of antibiotic agents."
Delayed skin reactions were noted in Moderna’s large clinical trial on its vaccine, occurring in less than 1% of receipts following the first dose and only in 0.2% after they received the second dose.
The doctors noted that all vaccine recipients went on to receive the second dose.
"Given that neither local injection-site reactions nor delayed-type hypersensitivity reactions are contraindications to subsequent vaccination, all 12 patients were encouraged to receive the second dose and completed their mRNA-1273 vaccination course," they wrote.
CLICK HERE FOR COMPLETE CORONAVIRUS COVERAGE
Speaking to Bloomberg, Kimberly Blumenthal, the lead author of the paper and co-director of the clinical epidemiology program at Massachusetts General Hospital in Boston, urged those who may experience a delayed skin reaction following the first dose to ensure they receive the second, as this specific reaction is not a danger, she said.
"Our aim was to show how dramatic they can be, while at the same time no one had it more severe with dose 2," she said. "So many of the patients I cared for with this were concerned about them. Is this an infection? (No!) Does this mean I cannot have dose 2? (No!) Will it happen with dose 2? (Not necessarily!)," she said.
"This is a nuisance, but it is not dangerous," added Blumenthal.