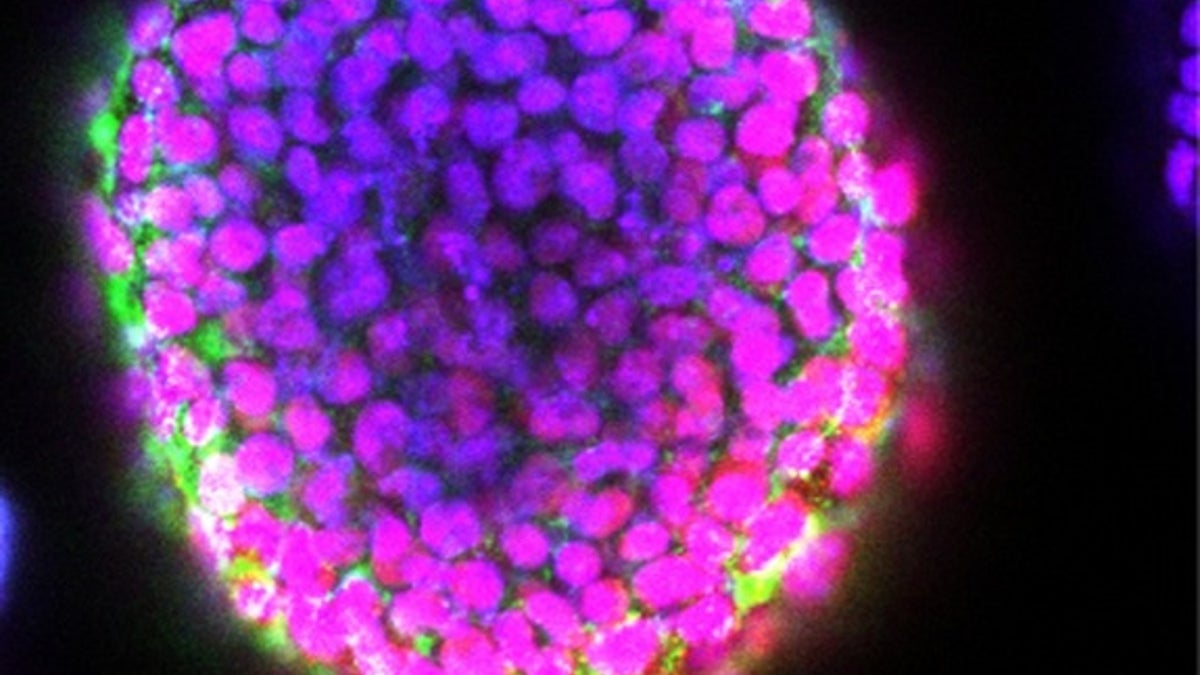
Researchers have figured out a way to print 3D blocks of living stem cells. (Wei Sun/Drexel University)
Gone are the days when 3D printers merely built plastic trinkets — scientists say 3D-printed structures loaded with embryonic stem cells could one day help doctors print out micro-organs for transplant patients.
Embryonic stem cells, obtained from human embryos, can develop into any kind of cell in the body, such as brain tissue, heart cells or bone. This property makes them ideal for use in regenerative medicine — repairing and replacing damaged cells, tissues and organs.
Scientists typically experiment with embryonic stem cells by dosing them with biological cues that guide them toward developing into specific tissue types — a process called differentiation. This process begins with the cells forming spherical masses called embryoid bodies — an activity that mimics the early stages of embryonic development. [7 Cool Uses of 3D Printing in Medicine]
Previous research suggested the best way to grow embryonic stem cells is not in flat lab dishes, but in 3D environments that mimic how these cells might develop in human bodies. Recently, scientists developed 3D printers for embryonic stem cells. A 3D printer works by depositing layers of material, just as ordinary printers lay down ink, except it can also lay down flat layers on top of one another to build 3D objects.
Until now, 3D printers for embryonic stem cells just generated flat arrays or simple mounds, called "stalagmites," of cells. Now, researchers say they have, for the first time, developed a way to print 3D structures laden with embryonic stem cells.
"We are able to apply a 3D-printing method to grow embryoid bodies in a controlled manner to produce highly uniform blocks of embryonic stem cells," study co-author Wei Sun, a professor of mechanical engineering at Tsinghua University in Beijing and Drexel University in Philadelphia,told Live Science.
In principle, these blocks could be used like Lego bricks to build tissues "and potentially even micro-organs," Sun added.
In experiments, the researchers simultaneously printed out mouse embryonic stem cells with a hydrogel, the same kind of material from which soft contact lenses are made. Because embryonic stem cells are relatively fragile, the scientists made sure to protect the cells as much as possible — for instance, by finding the most comfortable temperature for them and increasing the size of the nozzle used to print them out.
Ninety percent of the cells survived the printing process, according to the new study. The cells proliferated into embryoid bodies within the hydrogel scaffolds and generated the kind of proteins that would be expected from healthy embryonic stem cells, the researchers said. The scientists also noted that they could dissolve the hydrogel to harvest the embryoid bodies.
The size and uniformity of embryoid bodies can greatly influence what types of cells they become. The researchers said their new technique resulted in better control over embryoid body size and uniformity than previous methods could achieve.
"The grown embryoid body is uniform and homogenous, and serves as [a] much better starting point for further tissue growth," Sun said in a statement. "It was really exciting to see that we could grow embryoid bodies in such a controlled manner."
"Our next step is to find out more about how we can vary the size of the embryoid body by changing the printing and structural parameters, and how varying the embryoid body size leads to 'manufacture' of different cell types," study co-lead author Rui Yao an assistant professor at Tsinghua University in Beijing, said in a statement.
In the long term, the researchers would like to print different kinds of embryoid bodies side by side. "This would promote different cell types developing next to each other, which would lead the way for growing micro-organs from scratch within the lab," Yao said in a statement.
The scientists detailed their findings online Nov. 4 in the journal Biofabrication.
- The 10 Weirdest Things Created By 3D Printing
- In Images: Live Cells Printed Into Weird Shapes
- Magnificent Microphotography: 50 Tiny Wonders
Copyright 2015 LiveScience, a Purch company. All rights reserved. This material may not be published, broadcast, rewritten or redistributed.







































