Dr. Siegel: Access to insulin is the next supply chain crisis
Fox News' Dr. Marc Siegel discusses a recent study on marijuana users more likely to visit the ER and fears there will be supply chain shortages on insulin.
Most of the 38 million people living with diabetes in the U.S. use daily injections or insulin pumps to keep glucose at safe levels — but new research suggests that a third option could be just as effective.
In a study led by Dr. Irl B. Hirsch, M.D., medical director of the Diabetes Care Center of the University of Washington Medical Center, an inhaled form of insulin — similar to an asthma inhaler — worked just as well as injections or pumps to control type 1 diabetes.
The research was presented last week at the American Diabetes Association (ADA)’s 84th Scientific Sessions in Orlando, Florida.
The clinical trial tested a product called Afrezza, an inhaled insulin made by MannKind Corporation in California.
Afrezza, the only inhaled insulin on the market, has been available since getting FDA approval in June 2014.

An inhaled form of insulin worked just as well as injections or pumps to control type 1 diabetes in a recent study. (iStock/MannKind)
"The data presented at ADA demonstrates that inhaled insulin should be offered as a clear choice for adults living with type 1 diabetes," said Michael Castagna, PharmD, CEO of MannKind Corporation.
"We are also looking forward to releasing our pediatric trial data later this year."
Benefits of a third option
"In those with type 1 diabetes, insulin is required for survival," Hirsch told Fox News Digital in an interview.
"With continuous glucose sensing, glucose control has been dramatically improved — but not everyone reaches the target with multiple injections or pumps, and there are many pros and cons with each therapy," he said.
With pumps, people must wear the device, which can lead to skin problems.
They also have to purchase extra accessories.
Blood glucose levels can also drop with exercise, Hirsch warned, which can be problematic.

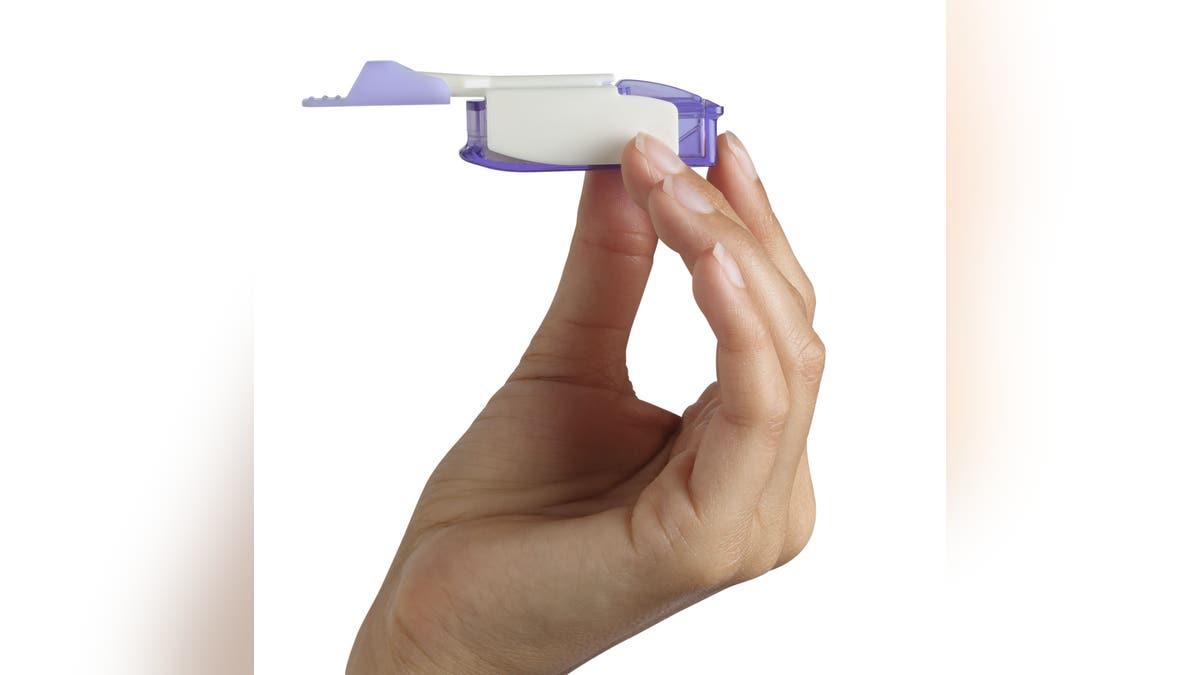
Afrezza, an inhaled insulin pictured here, is made by MannKind Corporation in California. (MannKind)
"Injections overall can be more convenient for some, but they don’t do as well as pump patients," he said.
With Afrezza, the product is inhaled into the lungs before meals, and the fast-acting insulin minimizes the glucose spike often seen after eating, Hirsch noted.
"Patients with type 1 diabetes should consider this as another option for their mealtime insulin, and talk to their doctor about this choice."
During the 17-week study, researchers evaluated the results of 141 adults who were assigned to either use the Afrezza inhaler or continue with traditional methods of injection or pump delivery.
At the 17-week mark, all participants switched to the inhaler for another 13 weeks.

Dr. Irl B. Hirsch, M.D., medical director of the Diabetes Care Center of the University of Washington Medical Center, led the new study. (MannKind)
All groups were assessed with continuous glucose monitoring at the start of the study, at 17 weeks and again at 30 weeks.
Among the inhaled insulin group, 30% of participants reached their target glucose levels (less than 7% blood sugar) compared to 17% of the people using injections and pumps.
There was no difference in hypoglycemia (low blood sugar) between the groups.
UTAH MOM FIGHTS FOR HER DAUGHTER’S ACCESS TO DISCONTINUED DIABETES MEDICATION: ‘LIFE-SAVING'
"In general, there was no difference in our primary endpoint, HbA1c, a reflection of average blood sugar," Hirsch said.
"But that alone is misleading — many patients did better with their glucose control, while others did worse."
With Afrezza, the product is inhaled into the lungs before meals, and the fast-acting insulin minimizes the glucose spike often seen after eating, a doctor said. (MannKind)
"The point is, inhaling insulin isn't for everyone, but some did better than they did on their pumps."
The people who saw the best results inhaled insulin between meals and at bedtime, Hirsch added.
CLICK HERE TO GET THE FOX NEWS APP
At the end of the study, more than half of the participants said they would opt to stay on the inhaled insulin therapy.
"The biggest takeaway is that patients with type 1 diabetes should consider this as another option for their mealtime insulin, and talk to their doctor about this choice," he recommended.
‘Adds value’
The American Diabetes Association acknowledged the promise of the study findings in an email to Fox News Digital.
"We look forward to our Scientific Sessions every year to see data like the INHALE-3 study’s findings, which have the potential to expand diabetes care," Raveendhara Bannuru, M.D., PhD, the ADA’s vice president of medical affairs and quality improvement outcomes in Boston, Massachusetts, told Fox News Digital via email.

"With continuous glucose sensing, glucose control has been dramatically improved," a doctor told Fox News Digital. (iStock)
"We are hopeful for the continuous development of alternative insulin delivery methods that could offer options for people living with diabetes," the group also said in the statement.
"The INHALE-3 trial demonstrated that inhaled insulin, combined with insulin degludec, effectively reduces A1c levels without increasing hypoglycemia or weight gain in people with type 1 diabetes. This adds value to the options in insulin therapy."
Potential risks and limitations
While more people met their glycemic targets with Afrezza, some subjects saw worse readings when switching from usual methods to inhaled insulin — "potentially due to missing doses of inhaled insulin during the day and/or underdosing going into bedtime," the researchers wrote.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
"We didn't see any concerns," Hirsch said when asked about side effects.
"As expected, a few people coughed immediately when dosing their insulin, but no major concerns were seen and everyone continued on their inhaled insulin."
"Not everyone reaches the target with multiple injections or pumps, and there are many pros and cons with each therapy," a doctor said. (iStock)
The most common side effects noted in the study were hypoglycemia, cough and throat pain or irritation.
Afrezza has been linked to a risk of acute bronchospasm in patients with chronic lung disease, such as asthma or COPD, according to the manufacturer.
For more Health articles, visit www.foxnews/health
Before starting Afrezza, patients should see a doctor for a physical examination and testing to measure lung function.
Patients who smoke or who recently quit smoking should not take the inhaled medication.