Doctor: The goal of the COVID vaccine is protection against serious illness
FDA vaccine advisory board member Dr. Paul Offit explains the ‘unrealistic expectation’ for vaccines to protect against mild illness.
Pfizer and BioNTech plan to submit a request for emergency use authorization for their COVID-19 vaccine in children six months to 5 years old as soon as Tuesday, which could set the vaccine up to be approved for young children in the coming weeks, the Washington Post reports.
The companies began a clinical trial last year to examine the effects of two doses of 3-microgram shots in children younger than 5.
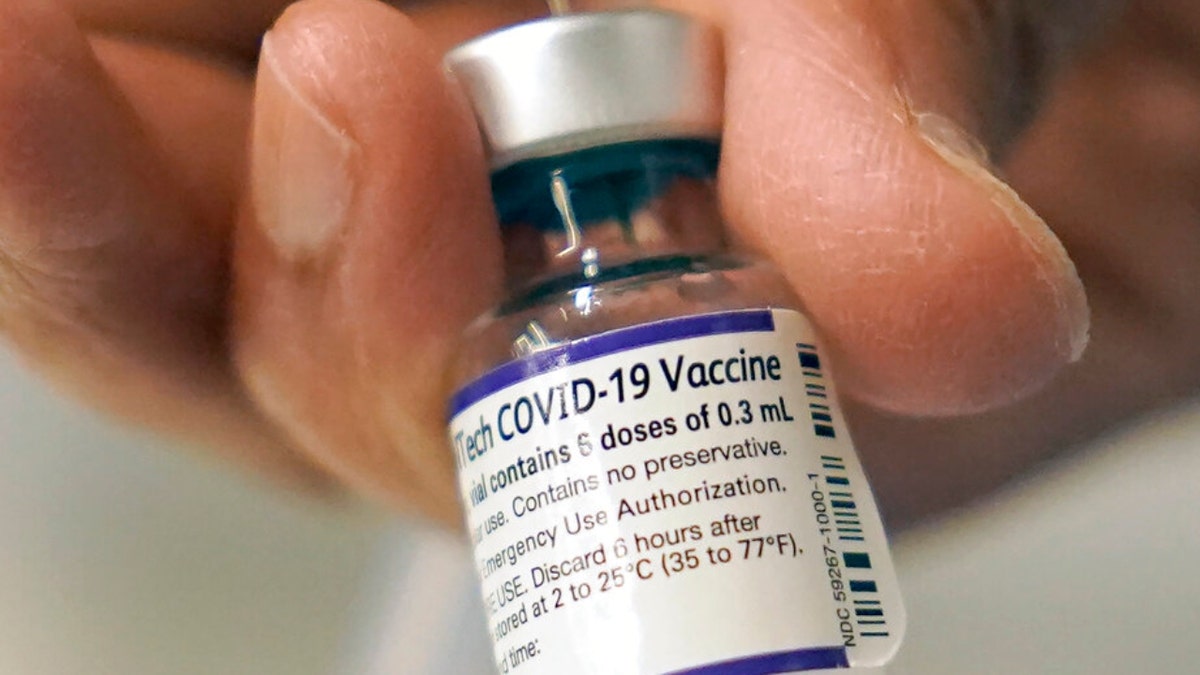
Dr. Manjul Shukla transfers Pfizer COVID-19 vaccine into a syringe, Thursday, Dec. 2, 2021, at a mobile vaccination clinic in Worcester, Mass. ((AP Photo/Steven Senne))
The vaccine is already approved for emergency use in children between the ages of 5 and 11, who receive 10 microgram doses. Children older than 12 and adults receive 30 microgram doses.
Two 3-microgram doses were found to be effective in children between six months and 2 years old, but children between the ages of 2 and 5 did not have the same immune response as the larger two-dose series in older children.

21/12/25: A tray with vials containing Pfizer/BioNTech COVID-19 booster vaccine seen at a vaccination center. ( Dinendra Haria/SOPA Images/LightRocket via Getty Images)
That finding prompted Pfizer to study the administration of a third 3-microgram dose two months after the last dose.
The FDA urged Pfizer and BioNTech to go ahead and submit the two-dose data so the approval process could begin, according to the Washington Post.
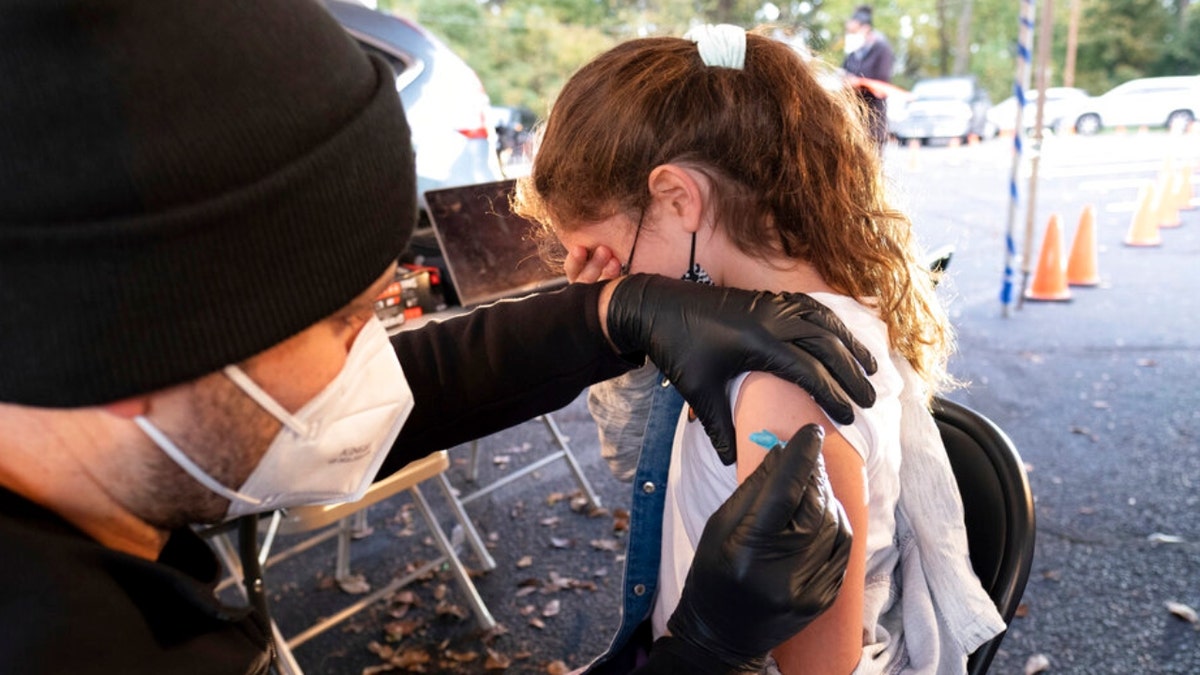
Leah Lefkove, 9, covers her face as her dad Dr. Ben Lefkove gives her the first COVID-19 vaccine at the Viral Solutions vaccination and testing site in Decatur, Georgia. (AP Photo/Ben Gray)
Pfizer's vaccine was authorized for children between the ages of 5 and 11 in late October.
About three months later, only 8.7 million children between the ages of 5 and 11 have received at least one dose and 6.2 million are fully vaccinated.
CLICK HERE TO GET THE FOX NEWS
There have been 11.4 million COVID-19 cases in children since the pandemic began, making up about 18.6% of all cases, but children only represent 1.6%-4.4% of all COVID-19 hospitalizations and 0.00%-0.25% of all COVID-19 deaths, according to the American Academy of Pediatrics.