FDA approves emergency use of antibody to treat COVID-19
Dr. Marc Siegel weighs in on ‘America’s Newsroom.’
A doctor in Maryland said he had to cancel potentially life-saving monoclonal antibody infusions for about 250 people over the last week after the federal government stopped distributing treatments made by Regeneron and Eli Lilly because they aren't effective against omicron, even though the delta variant, which the drugs are effective at treating, was still dominant at the time.
The Office of the Assistant Secretary for Preparedness and Response halted the allocation of those two antibody treatments last Thursday amid the rise of omicron, which the CDC had said days earlier was responsible for 73.2% of all new cases.
But the CDC backtracked on that alarming estimate this week, revising it down to just 22.5% for the week ending Dec. 18, more than a 50-point drop.
The delta variant, which Regeneron and Eli Lilly's treatments are effective against, was actually responsible for 77% of all new cases when the federal government stopped distributing those antibody drugs.
Now, one doctor says the government's massive miscalculation cost lives.
"I am as angry as I possibly can be about this," Dr. Ron Elfenbein, the medical director and CEO of FirstCall Medical Center in Gambrills, Maryland, told Fox News Digital on Wednesday.
"The fact that these people are so adamant that they're right when they're using faulty data and they're using faulty logic and frankly statistical modeling that has never been correct, ever, throughout this entire pandemic, to look at this, is just beyond the pale…. People are definitely going to die because of this or need hospitalization because [health officials] misread the statistics."
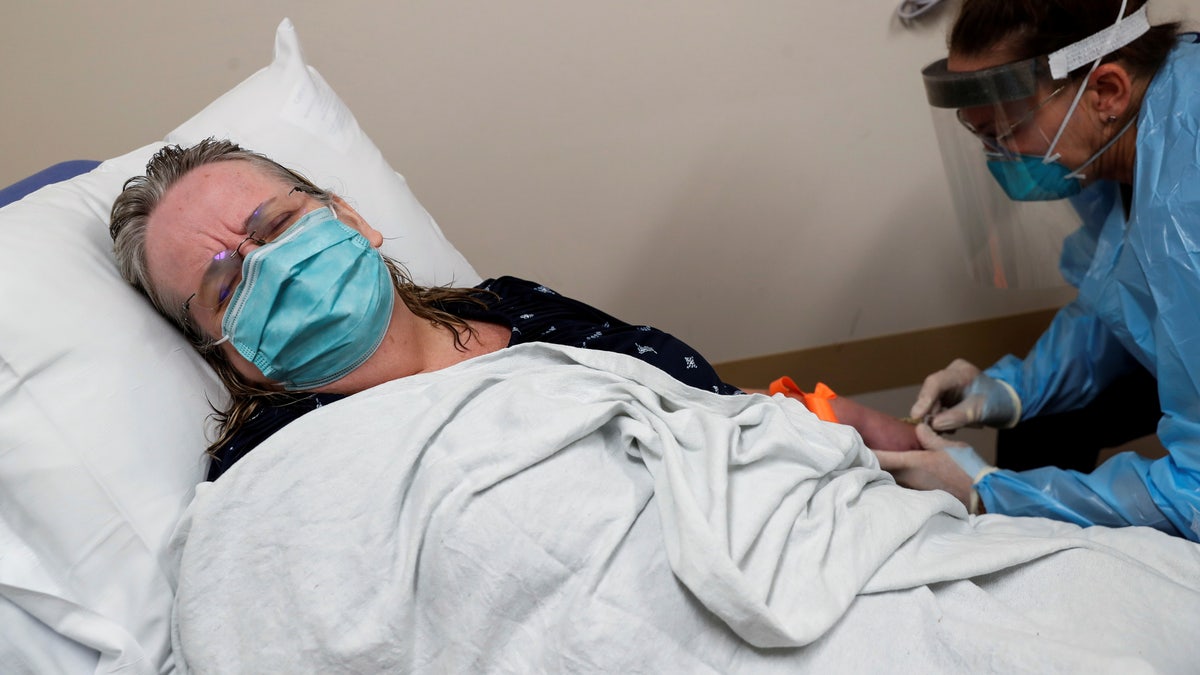
A registered nurse administers the Regeneron monoclonal antibody to a patient (Reuters/Shannon Stapleton)
The FDA has authorized three different monoclonal antibody treatments, which are lab-made substitutes for antibodies that can help fight off viruses.
The two treatments made by Regeneron and Eli Lilly are ineffective against omicron, according to the U.S. Department of Health and Human Services, but a third treatment created by British drugmaker GlaxoSmithKline has shown promise at beating back the new variant.
REGENERON REPORTS COVID-19 ANTIBODY COCKTAIL REDUCED INFECTION RISK BY 82% FOR EIGHT MONTHS
When the federal government stopped the allocation of Regeneron and Eli Lilly's drugs last week, the Maryland Department of Health sent a letter to doctors throughout the state that said "sites… have been directed to stop administering these therapeutics."
"Maryland is experiencing a sharp rise in COVID-19 Omicron variant cases. It is now the most dominant variant in the state," Maryland's top health officials wrote to doctors in a Dec. 23 letter, which was obtained by Fox News Digital.

A Regeneron monoclonal antibody infusion bag (Joe Cavaretta/South Florida Sun-Sentinel via AP, File)
Now that the CDC has significantly lowered its estimate for how prevalent omicron was at the time, Dr. Elfenbein said the pause in treatments was clearly unnecessary and is "absolutely hurting people."
"I don't know how many people throughout the country are dead, dying, in the hospital, or about to be hospitalized because of the mistakes that they just made," Dr. Elfenbein, who runs two monoclonal antibody clinics, said on Wednesday.
"It's just the height of bureaucratic arrogance, and it's just, it's horrible. I had to turn friends away, family, people call me, ‘Oh, my uncle has cancer. Can he get an infusion?’ I'm like, 'I cannot give you an infusion because I will lose my medical license.'"
ASTRAZENECA TRIALS SHOW ANTIBODY DRUG MORE THAN 80% EFFECTIVE AT PREVENTING COVID-19
The Office of the Assistant Secretary for Preparedness and Response said in an update on Wednesday afternoon that they will still pause allocations of Regeneron and Eli Lilly's drugs for states that have greater than 80% prevalence of omicron, but will restart allocations for states below that 80% threshold.
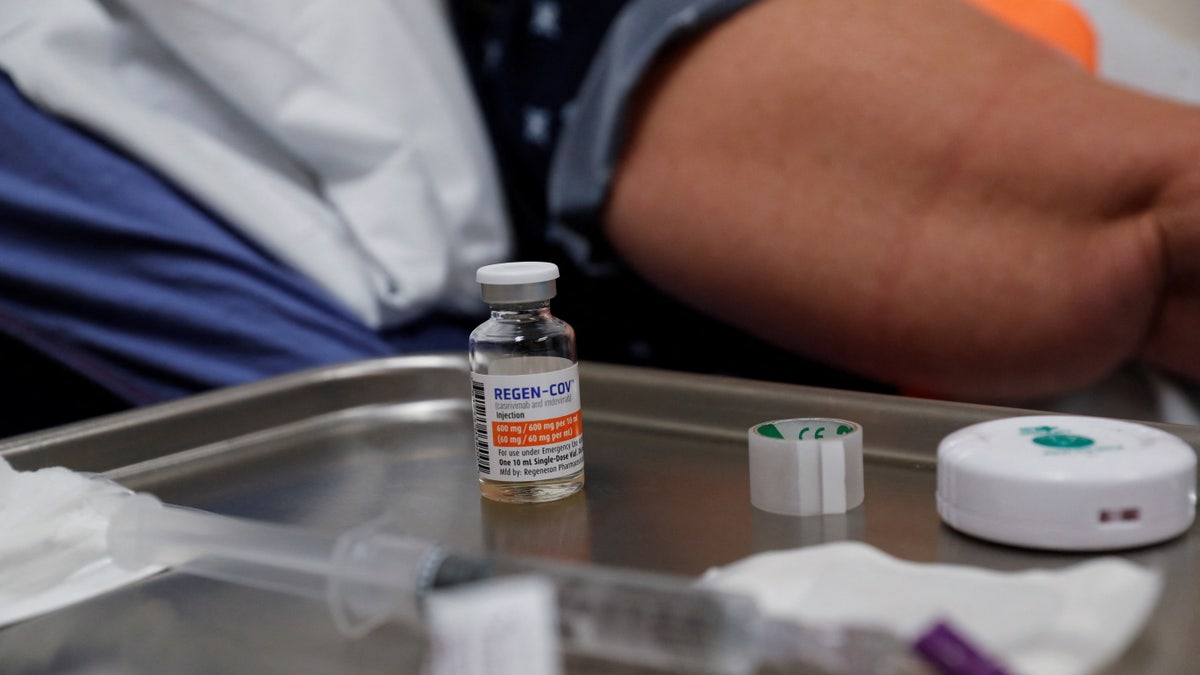
A vial of Regeneron monoclonal antibody sits on a medical table. (Reuters/Shannon Stapleton)
If treatment sites are able to distinguish between variants using testing, then they can still use Regeneron and Eli Lilly's drugs, even in states that are above 80% omicron prevalence, the federal government added.
Omicron is responsible for 57.7% of new cases in Maryland's region, which includes Delaware, Pennsylvania, Washington, D.C., Virginia, and West Virginia, according to the latest CDC estimate.
Nationwide, omicron was responsible for 58.6% of all new cases in the week that ended Dec. 25, narrowly edging out the delta variant.
CLICK HERE TO GET THE FOX NEWS APP
GlaxoSmithKline's antibody treatment, sotrovimab, is effective against COVID-19, but states nationwide are experiencing shortages of the lifesaving drug.
"Physicians are strongly encouraged to prioritize the use of sotrovimab for individuals who are 65 years old and older and individuals who are moderately to severely immunocompromised," the Maryland Department of Health told doctors last week.



