Fox News Flash top headlines for June 4
Fox News Flash top headlines are here. Check out what's clicking on Foxnews.com.
Fitbit announced on Wednesday that it has become the latest entity to be granted emergency use approval by the FDA to help with the coronavirus pandemic, getting the nod for a new ventilator.
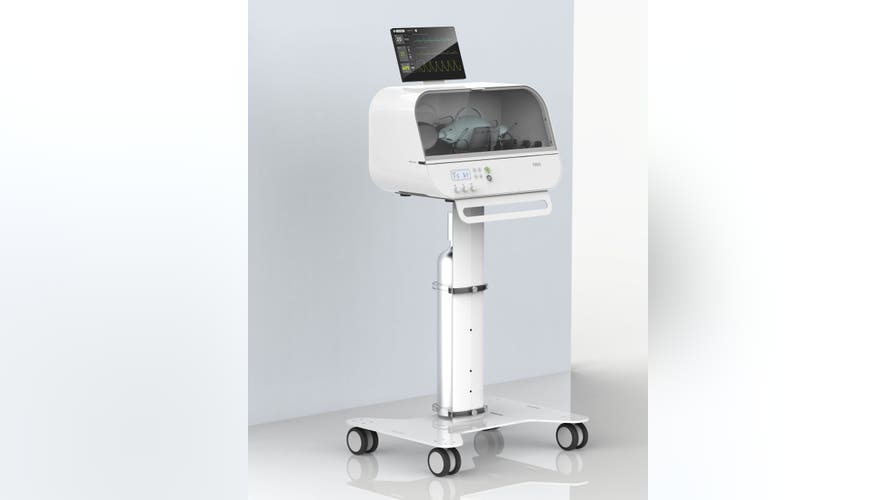
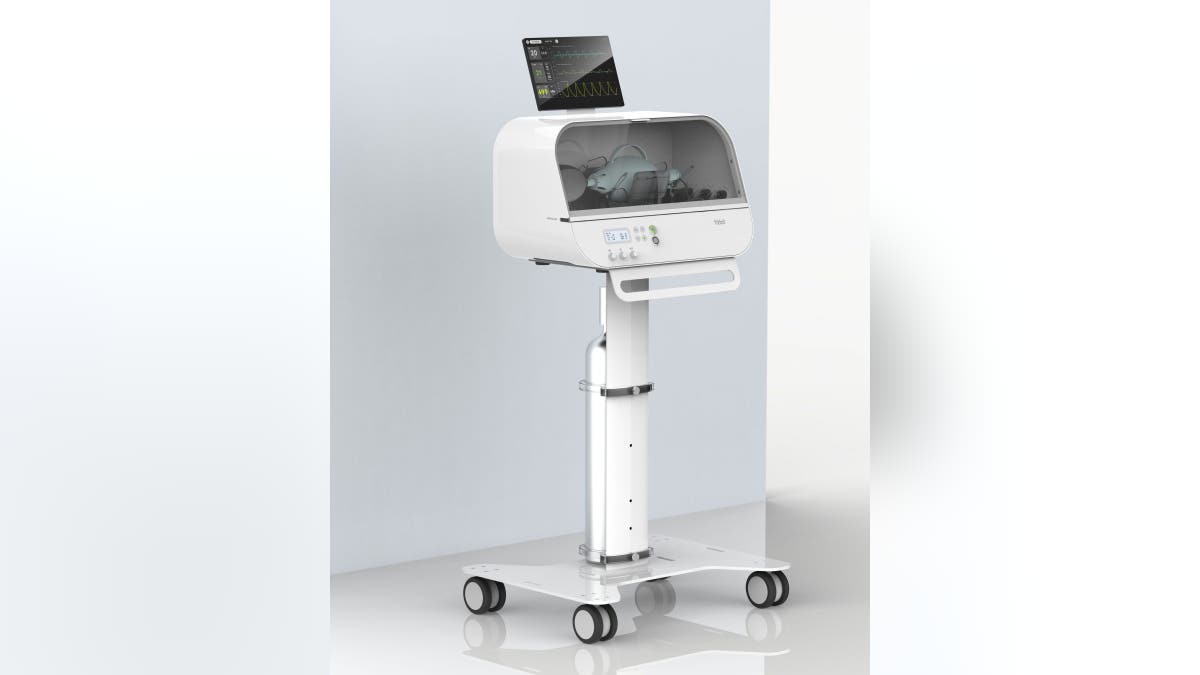
Known as the Fitbit Flow, the "low-cost" ventilator is based on the MIT E-Vent Design toolbox and Rapidly Manufactured Ventilation Systems. The consumer tech company, known for its wearable devices, consulted with Oregon Health & Science University emergency medicine clinicians and the Mass General Brigham Center to design and create the product specifically for COVID-19.
“COVID-19 has challenged all of us to push the boundaries of innovation and creativity, and use everything at our disposal to more rapidly develop products that support patients and the health care systems caring for them,” said James Park, co-founder and CEO of Fitbit, in a statement. “We saw an opportunity to rally our expertise in advanced sensor development, manufacturing, and our global supply chain to address the critical and ongoing need for ventilators and help make a difference in the global fight against this virus.”
(Credit: Fitbit)
CORONAVIRUS VENTILATOR DEVELOPED BY NASA APPROVED BY FDA FOR EMERGENCY USE
It's unclear exactly how much the Fitbit Flow will cost, but the company told The Verge it aims to sell it around for $5,000.
Fox News has reached out to Fitbit with a request for clarification.
The Fitbit Flow is not a replacement for traditional ventilators, which can cost as much as $20,000 to $30,000. Its design was based on standard resuscitator bags that are used by paramedics, as well as "sophisticated instruments, sensors, and alarms that work together to support automated compressions and patient monitoring."
By comparison, the NASA-designed ventilator would cost roughly between $2,000 and $3,000, according to Business Insider. It was granted EUA approval by the FDA in late April, Fox News previously reported.
Engineers at NASA's Jet Propulsion Laboratory in Pasadena, Calif., built the device, known as VITAL (Ventilator Intervention Technology Accessible Locally), in just 37 days, using a fraction of the parts a traditional ventilator uses.
It's unclear how large the shortage of ventilators is in the U.S. and around the world, but the need for additional machines is clear, though not as dire as initially feared.
According to a paper published in late April in the New England Journal of Medicine, the range of ventilators in the U.S. ranged from 60,000 to 160,000 at the time.
The U.S. has a national strategic reserve of ventilators, but that has been criticized as being insufficient to fill the needs of hospitals around the country.
COVID-19 PATIENTS ARE NOT INFECTIOUS 11 DAYS AFTER GETTING SICK, STUDY SUGGESTS
In early May, The Wall Street Journal reported that manufacturers, including General Electric and Medtronic, delivered 4,200 ventilators to the national stockpile.
CLICK HERE FOR COMPLETE CORONAVIRUS COVERAGE
As of Thursday morning, more than 6.5 million coronavirus cases have been diagnosed worldwide, more than 1.85 million of which are in the U.S., the most impacted country.