Fox News Flash top headlines for April 29
Fox News Flash top headlines are here. Check out what's clicking on Foxnews.com.
Get all the latest news on coronavirus and more delivered daily to your inbox. Sign up here.
The National Institutes of Health is launching a new initiative to turbocharge innovation, development and commercialization of COVID-19 testing technologies as a way to help society get back to a place where restrictions can be lifted.
Amid the global pandemic, which has infected 1,028,217 in the U.S. and killed at least 59,466, this new Rapid Acceleration of Diagnostics (RADx) initiative will use money from the federal stimulus package to advance technologies that would speed up the development of rapid and widespread COVID-19 testing.
“We need all innovators, from the basement to the boardroom, to come together to advance diagnostic technologies, no matter where they are in development,” said NIH Director Francis S. Collins in a press release. “Now is the time for that unmatched American ingenuity to bring the best and most innovative technologies forward to make testing for COVID-19 widely available.”
'DEVASTATINGLY' EFFECTIVE CORONAVIRUS CAPTURED IN IMAGE OF CELL
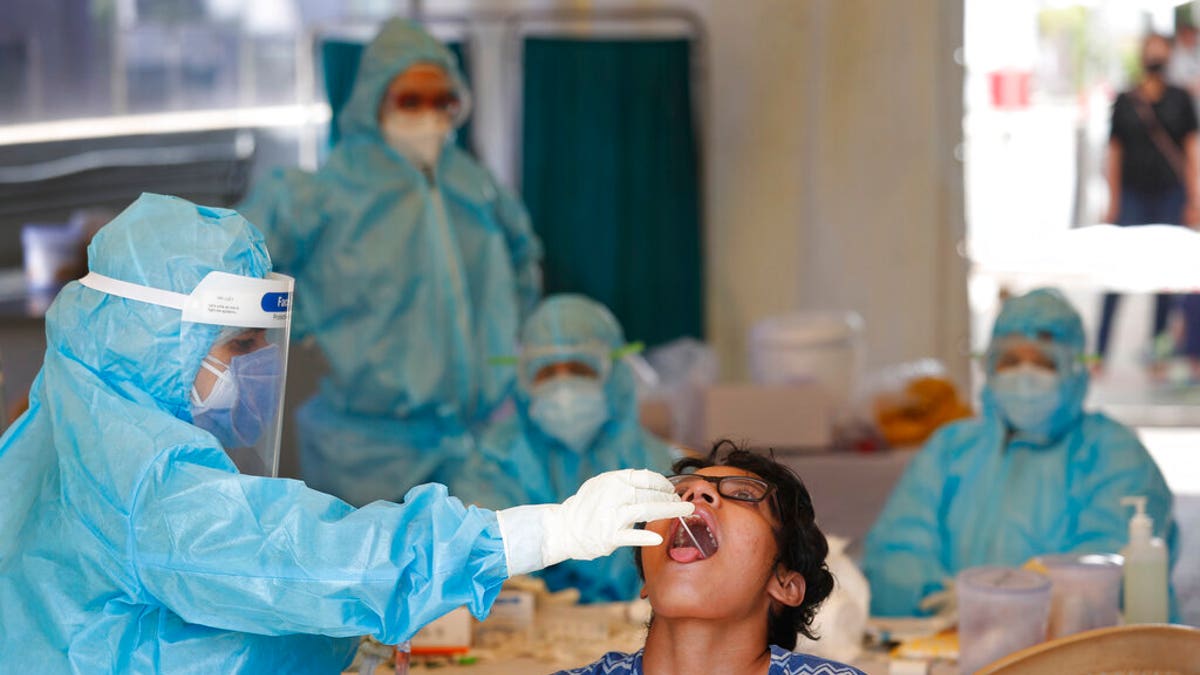
The NIH wants to rapidly scape up the production of safe, accessible COVID-19 tests in the United States. Above, a health worker takes a swab test at a COVID-19 testing center in New Delhi, India, Monday, April 27, 2020.(AP Photo/Manish Swarup)
NIH wants all scientists and inventors with a rapid testing technology to compete in a national COVID-19 testing challenge for their share of up to $500 million over all phases of development.
According to the NIH announcement, there will be a very competitive there-stage selection process for all of the technologies to determine which candidates are best for at-home or point-of-care tests. The finalists will receive assistance, for technical, manufacturing and business aspects, from experts.
The challenge has a lofty goal: to make millions of accurate, easy-to-use tests per week available for all Americans by the end of this summer, with even more in time for the flu season.
“Americans are innovators and makers,” said Bruce J. Tromberg, director of NIH’s National Institute of Biomedical Imaging and Bioengineering (NIBIB), in a press statement. “We need American tech experts, innovators and entrepreneurs to step up to one of the toughest challenges we’ve faced as a country, to help get us safely back to public spaces.”
WHY ARE CHILDREN LESS AFFECTED BY CORONAVIRUS?
NIH will work in close consultation with the U.S. Food and Drug Administration, the Centers for Disease Control and Prevention and the Biomedical Advanced Research and Development Authority (BARDA) to advance these goals.