Fox News Flash top headlines for December 17
Fox News Flash top headlines are here. Check out what's clicking on Foxnews.com.
Sen. Dick Durbin, D-Ill., a top critic of President Trump, gave the administration praise as a second COVID-19 vaccine tied to Operation Warp Speed is expected to receive emergency use authorization from the FDA.
"The Warp Speed project appears to be a dramatic success," Durbin said on the Senate floor on Thursday. "I pray that it will be. Although I’ve been a frequent critic of this administration, I want to give them credit for organizing this effectively, and delivering a vaccine in a timely way, almost amazing timely way in this pandemic that we face."
ALEX AZAR DETAILS PFIZER COVID-19 VACCINE ROLLOUT 'HICCUPS'
"I thank all who were involved in it, especially the scientists and researchers who didn’t give up until they found these vaccines," he continued.
Earlier in the year, Durbin cast doubt on Trump's efforts to ensure the development of a vaccine before 2021. In September, Durbin accused Trump of pressuring companies to rush a vaccine and "undermin[ing] credibility in this process."
"President Trump has clearly staked his campaign reelection strategy on the approval of a vaccine for this virus," Durbin wrote in letters to pharmaceutical companies, including Moderna and Sanofi. "I write today to assess, in your important efforts to quickly bring a safe and effective vaccine to the public, how your company plans to maintain scientific and data-based standards in the face of White House political pressure regarding the approval process."
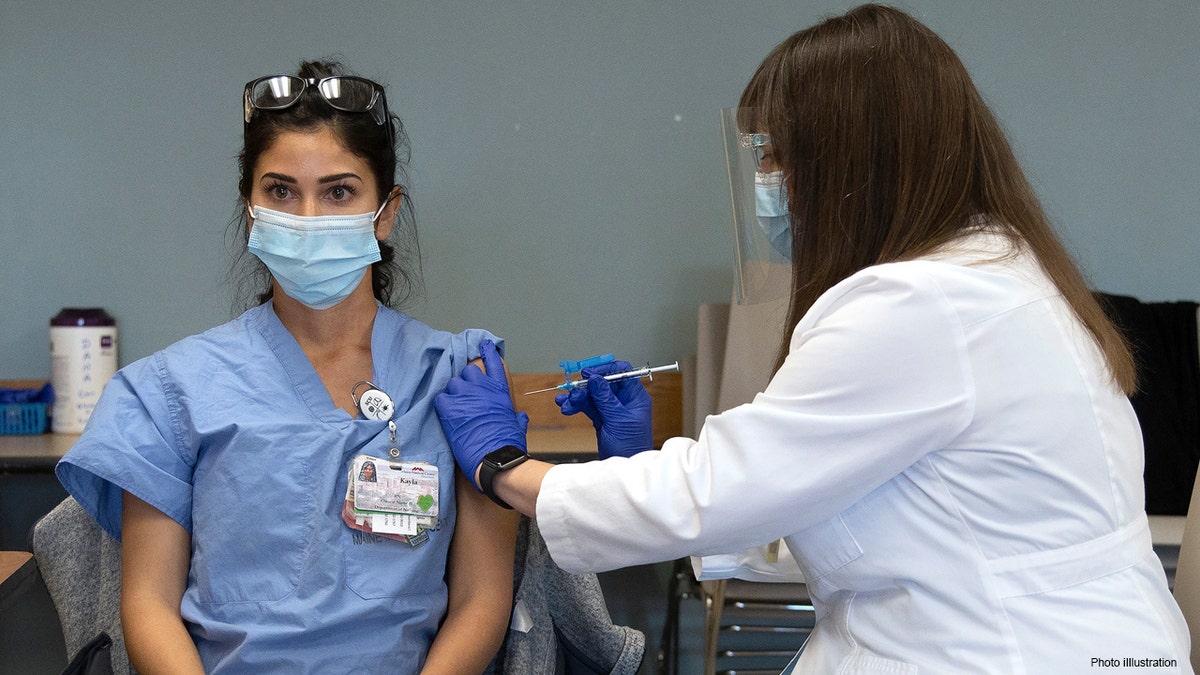
Nurse Kayla Mitchell, left, of Maine Medical Center’s COVID ICU unit in Portland, Maine, becomes the first person in the state to receive the Pfizer-BioNTech COVID-19 vaccine, Tuesday, Dec. 15, 2020. (Derek Davis/Portland Press Herald via AP)
It's possible the U.S. could have four coronavirus vaccines with FDA approval by February or March as Moderna, Johnson & Johnson and AstraZeneca approach the finish line, Health and Human Services Secretary Alex Azar told "Varney & Co." on Monday. The Pfizer-BioNTech coronavirus vaccine candidate received emergency authorization earlier in December.
CLICK HERE TO GET THE FOX NEWS APP
"That’s the genius of Operation Warp Speed," Azar said. "We spread our bets around not just multiple companies, but multiple platforms, technologies of vaccines. Moderna, which we're incredibly excited about, if everything's on track, we could see approval this week."
The U.S. government will acquire 200 million doses of Moderna's coronavirus vaccine candidate, and Moderna received $4.1 billion in federal funding for the vaccine, including development, clinical trials and manufacturing, according to the Department of Health and Human Services website.





