Stem cell therapy shows promise for multiple sclerosis
{{#rendered}} {{/rendered}}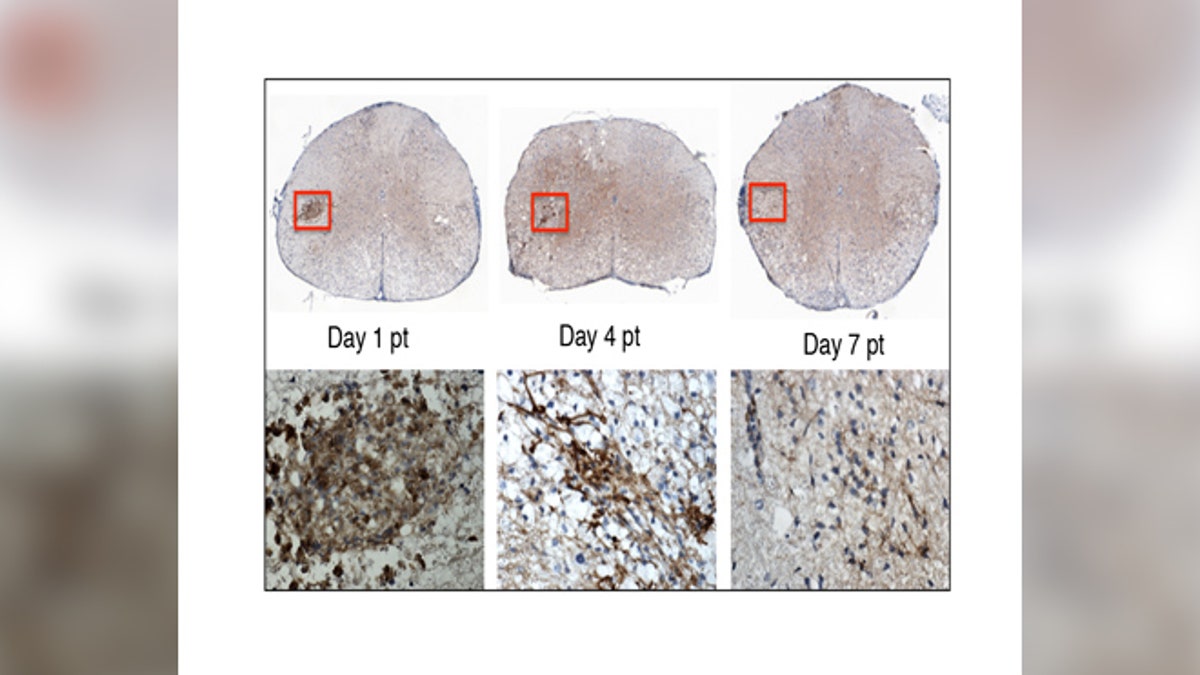
In this image, the top row shows the stem cells transplanted into the mouse spinal cord. The lower row shows a close-up of the stem cells (brown). By day 7 post-transplant, the stem cells are no longer detectable. Within this short period of time, the stem cells have sent chemical signals to the mouse’s own cells, enabling them to repair the nerve damage caused by MS. (image: Lu Chen)
For patients with multiple sclerosis (MS), current treatment options only address early-stage symptoms of the debilitating disease. Now, new research has found a potential treatment that could both stop disease progression and repair existing damage.
In a study published in Stem Cell Reports, researchers utilized a group of paralyzed mice genetically engineered to have an MS-like condition. Initially, the researchers set out to study the mechanisms of stem cell rejection in the mice. However, two weeks after injecting the mice with human neural stem cells, the researchers made the unexpected discovery that the mice had regained their ability to walk.
“This had a lot of luck to do with it; right place, right time” co-senior author Jeanne Loring, director of the Center for Regenerative Medicine at The Scripps Research Institute in La Jolla, California, told FoxNews.com. “[co-senior author Tom Lane] called me up and said, ‘You’re not going to believe this.’ He sent me a video, and it showed the mice running around the cages. I said, ‘Are you sure these are the same mice?’”
{{#rendered}} {{/rendered}}Loring, whose lab specializes in turning human stem cells into neural precursor cells, or pluripotent cells, collaborated with Tom Lane, a professor of pathology at the University of Utah whose focus is on neuroinflammatory diseases of the central nervous system. The team was interested in stem cell rejection in MS models in order to understand the underlying molecular and cellular mechanisms contributing to rejection of potential stem cell therapies for the disease.
Multiple sclerosis is an autoimmune disease that affects more than 2.3 million people worldwide. For people with MS, the immune system misguidedly attacks the body’s myelin, the insulating coating on nerve fibers.
“In a nutshell, it’s the rubber sheath that protects the electrical wire; the axon that extends from the nerve’s cell body is insulated by myelin,” Lane, who began the study while at the University of California, Irvine, told FoxNews.com
{{#rendered}} {{/rendered}}Once the myelin has been lost, nerve fibers are unable to transmit electric signals efficiently, leading to symptoms such as vision and motor skill problems, fatigue, slurred speech, memory difficulties and depression.
The researchers’ inadvertent treatment appeared to work in two ways. First, there was a decrease of inflammation within the central nervous system of the mice, preventing the disease from progressing. Secondly, the injected cells released proteins that signaled cells to regenerate myelin and repair existing damage.
While the stem cells were rejected in the mice after 10 days, researchers were able to see improvements for up to six months after initial implantation.
{{#rendered}} {{/rendered}}“One of the big hurdles in stem cell transplant therapies is rejection. What our data would argue, [is immunosuppression] may be a moot point because it doesn’t matter if [stem cells] are rejected, because they are, but we still see clinical improvement,” Lane said.
Now the team is working on figuring out how to use the stem cell-secreted proteins, or something that mimics the proteins, for clinical use— instead of transplanting stem cells directly, which would be more difficult both in creation and practical application. Additionally, drugs they create could get FDA approval more easily, compared to stem cell therapy, Loring said.
“Rather than delivering stem cells for MS, we want to deliver things stems cells make that are good for mice and presumably humans,” Loring said.
{{#rendered}} {{/rendered}}Currently, FDA-approved treatments for MS only offer help for patients with the relapsing-remitting form of the disease, in which MS symptoms go away and reappear in patients over unpredictable intervals of time. Over time, the intervals in which the disease is clinically silent shorten and a majority of people go on to develop progressive MS.
Existing drug treatments are designed to impede the activated immune cells from gaining access into the central nervous system and causing further damage. According to the National Multiple Sclerosis Society, it’s too soon to tell whether disease-modifying therapies alter or delay the transition to progressive MS, which is diagnosed when disease symptoms worsen. Before drugs were available, studies showed that 50 percent of those with relapsing-remitting MS would transition to progressive MS within 10 years, and 90 percent within 25 years.
“The problem is this—first off, the damage is already there, how do you repair the damage and potentially improve the clinical outcome for those patients?” Lane said. “Equally, if not more important, for people who have the progressive form, there’s nothing available to them for stopping it.”
{{#rendered}} {{/rendered}}This new research, funded by the National Multiple Sclerosis Society, may provide a two-prong solution of stopping disease progression and also allowing new myelin to be created. The next step for the team is to continue working to identify the stem cell secretion that has this beneficial effect. After continuing to work on animal models, they hope to go to clinical trial in the next 3 to 5 years.
“It’s a terrible disease,” Lane said. “That’s why we just want to stop it and want these people to live a normal life.”
