Fox News Flash top headlines for May 5
Fox News Flash top headlines are here. Check out what's clicking on FoxNews.com.
Get all the latest news on coronavirus and more delivered daily to your inbox. Sign up here.
The first doses of a coronavirus vaccine have been injected into human patients, Pfizer Inc. and BioNTech announced on Tuesday.
The first dosing of the BNT162 vaccine program began in Germany last week, according to a company statement. The trial phase aims to enroll up to 360 patients, ages 18 to 55. Once the younger group produces sound evidence of safety and immunogenicity, testing in older adults, or those between the ages of 65 and 85, will begin.
CLICK HERE FOR FULL CORONAVIRUS COVERAGE
“With our unique and robust clinical study program underway, starting in Europe and now the U.S., we look forward to advancing quickly and collaboratively with our partners at BioNTech and regulatory authorities to bring a safe and efficacious vaccine to the patients who need it most,” said Albert Bourla, Pfizer CEO and chairman, in the statement.
Sites dosing participants include NYU Grossman School of Medicine and the University of Maryland School of Medicine.
POSSIBLE CORONAVIRUS VACCINE ENTERS HUMAN TESTING TRIAL
University of Rochester Medical Center/Rochester Regional Health and Cincinnati Children’s Hospital Medical Center will start “enrollment shortly,” according to the company statement.
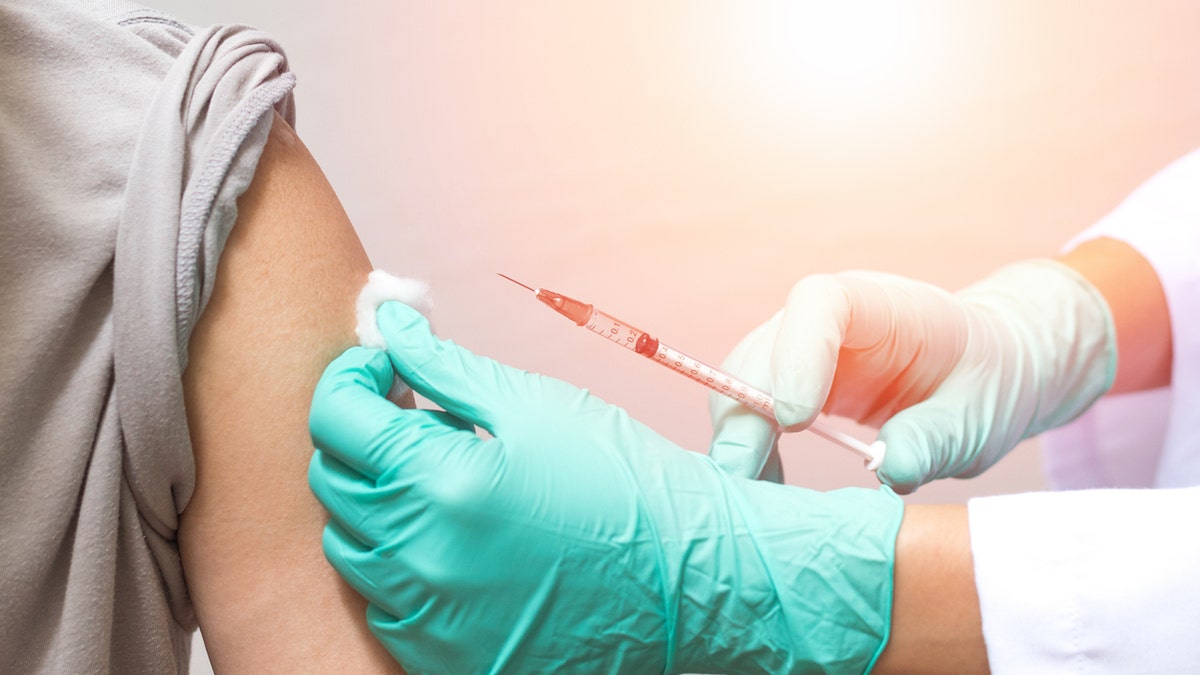
Pfizer Inc. and BioNTech announced the start of coronavirus vaccine trials in human patients. In anticipation of success, production has been scaled for a global supply. (iStock)
The development program includes four vaccine candidates, each of which is a different combination of a messenger RNA (mRNA) format, important in protein synthesis, and target antigen.
The trial will evaluate the varying vaccine candidates to determine which results in the safest, most effective vaccine.
With the anticipation of success, Pfizer and BioNTech are scaling up production for global supply, according to the company statement. The program should allow for the production of millions of vaccine doses in 2020, with hundreds of millions in production the following year.
CLICK HERE TO DOWNLOAD THE FOX NEWS APP
Manufacturing locations selected so far include Pfizer-owned sites in Massachusetts, Michigan and Missouri as well as Puurs, Belgium, with more to come. BioNTech will contribute toward production capabilities through sites in Mainz and Idar-Oberstein, Germany.
There are 102 potential COVID-19 vaccines in development as of April 30, according to the World Health Organization. Eight of the contenders have been approved for clinical trials.





















