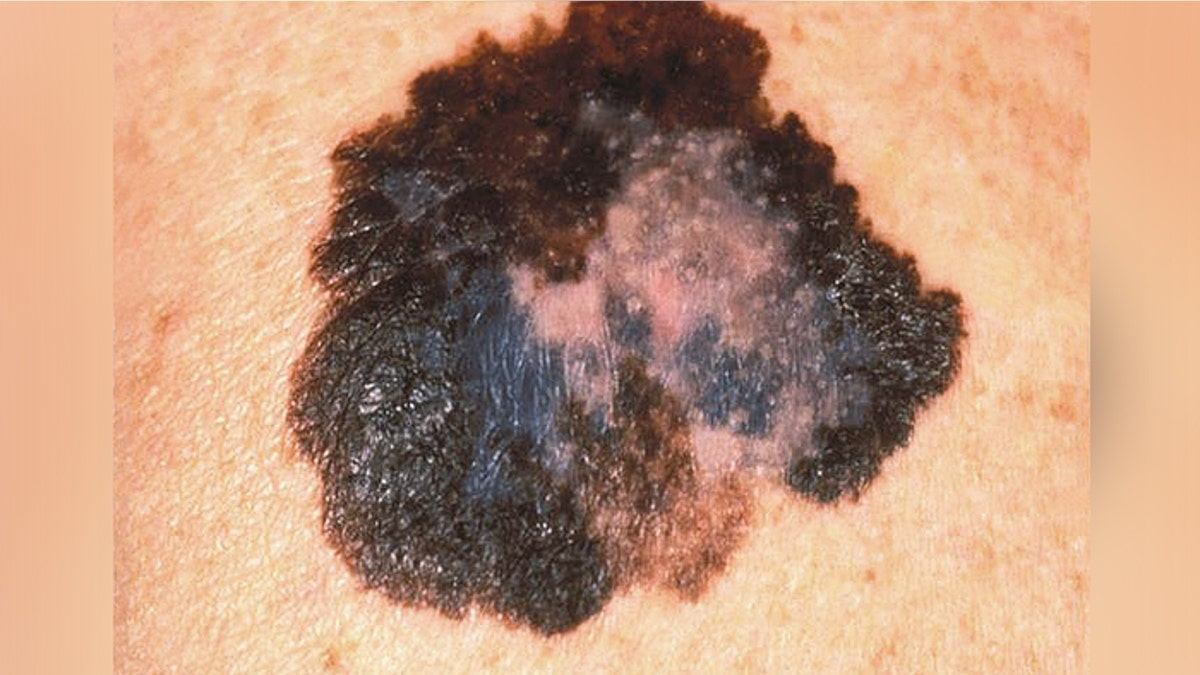
This image depicts the gross appearance of a cutaneous pigmented lesion, which had been diagnosed as superficial spreading malignant melanoma (SSMM). (CDC.gov)
Researchers have discovered a new biomarker that can predict whether melanoma patients with mutations in the BRAF gene will respond to certain kinds of cancer medications.
The discovery could help better guide treatment for patients with the disease, as they are often subjected to ineffective therapies to which their bodies are resistant.
The BRAF gene has been found to be an effective drug target for treating melanoma cancers, and the Food and Drug Administration (FDA) has approved two drugs inhibiting this gene. However, patients with melanomas harboring mutations in the BRAF gene are sometimes resistant to these medications, and most people who initially respond to BRAF-targeting therapies eventually relapse, as their tumors become resistant to the drugs.
While BRAF mutations are present in only 7 percent of all human cancers, they are seen in approximately 50 percent of melanomas. And there’s no concrete way of knowing which patients with BRAF mutations will respond to BRAF-targeting treatments and which ones will not.
“For virtually untreatable cancers, we’re seeing response rates of 60 to 80 percent with BRAF-targeting drugs.” Dr. Ryan Corcoran, a clinical investigator and assistant professor at the Massachusetts General Hospital Cancer Center and Harvard Medical School in Boston, Mass., told FoxNews.com. “…But 60 to 80 percent response rate means there are still 20 to 40 percent of patients who don’t respond. Determining which patients are most likely to respond (to these drugs) and perhaps should be diverted to an alternative therapy could be really beneficial.”
Corcoran said that previous research has identified numerous mechanisms behind the resistance to BRAF inhibitors, but it can be a daunting task screening patients for these mechanisms. So he and his team decided to analyze them further.
“We thought, ‘Perhaps all these resistance mechanisms converge into a key downstream pathway,’” Corcoran said. “Then maybe we could get a more universal yes-or-no predictor to see if patients respond to the drugs.”
After analyzing genetic models in the lab, Corcoran and his team found that the signaling pathway TORC1 was a key converging point for these mechanisms and that the presence of a protein called S6 could serve as a good predictor of sensitivity to BRAF-inhibiting medication.
“There’s a protein substraight called S6, which is involved in protein translation in the cell,” Corcoran said. “It’s basically used as an indicator of TORC1 activity. When the pathway is active, the presence of S6 is elevated, and vice versa.”
In their models, the researchers found that melanoma cancers that were sensitive to BRAF inhibitors saw a suppression of S6 after treatment. In contrast, almost all melanoma cancers that were resistant to the drugs maintained higher levels of S6 post treatment.
To further verify this finding, the researchers collected tumor biopsies from nine melanoma patients, which had been taken before and after treatment with BRAF-targeting drugs. The found that one subset of patients had an effective shutdown of S6 after treatment, compared to the other patients who had maintained levels of S6 after treatment. Ultimately, the group of patients with effective suppression of S6 had a more than fivefold increase in repression-free survival compared to the those who had maintained S6.
“That suggests that if we can identify early on these patients who down regulate or fail to down regulate S6, we can predict who is most likely to respond,” Corcoran.
Applying this new knowledge, Corcoran and his team developed a new technique that can rapidly monitor, in real time, the levels of S6 in tumor cells. Using fine-needle aspiration biopsies from melanoma patients before and during the first couple weeks of their treatment with BRAF-targeting drugs, they could assess the activity of the S6 signaling pathway and quickly determine a patient’s resistance.
“It’s still in concept phase, but …if you have a way of determining (resistance), you can spare (a patient) unnecessary treatment,” Corcoran said. “By identifying this subgroup, you can do that at a much earlier point, rather than waiting two months, as is done, for a repeat CT scan. So this is really a way to guide treatment more rapidly and divert more patients to more effective therapy.”
The research was presented at the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics.
