Eric Swalwell, David Frum float 'despicable' new coronavirus vaccine mandates
The panel on 'The Five' offer reaction and analysis after former Bush aide speaks out against unvaccinated Americans
A low dose of the Pfizer-BioNTech coronavirus vaccine failed to produce an adequate response in children aged two to five years of age.
The companies announced the results from ongoing critical trials on Friday and said that after testing children 6 months to 5 years of age with one-tenth of the adult dose, children between 6 months and 2 years produced an immune response similar to people aged 16 to 25 after two doses but children between 2 and 5 did not.

A syringe is prepared with the Pfizer COVID-19 vaccine at a clinic in the Norristown Public Health Center in Norristown, Pa., Tuesday, Dec. 7, 2021. (AP Photo/Matt Rourke)
BIDEN WARNS OF 'WINTER OF SEVERE ILLNESS AND DEATH' FOR THE UNVACCINATED
"The companies will amend the clinical study evaluating the safety, tolerability, and immunogenicity of the Pfizer-BioNTech COVID-19 Vaccine in children 6 months to under 5 years of age," the statement said. "The study will now include evaluating a third dose of 3 µg at least two months after the second dose of the two-dose series to provide high levels of protection in this young age group."
The companies said if the three-dose study is successful, they plan to apply for emergency authorization sometime in the first half of 2022.
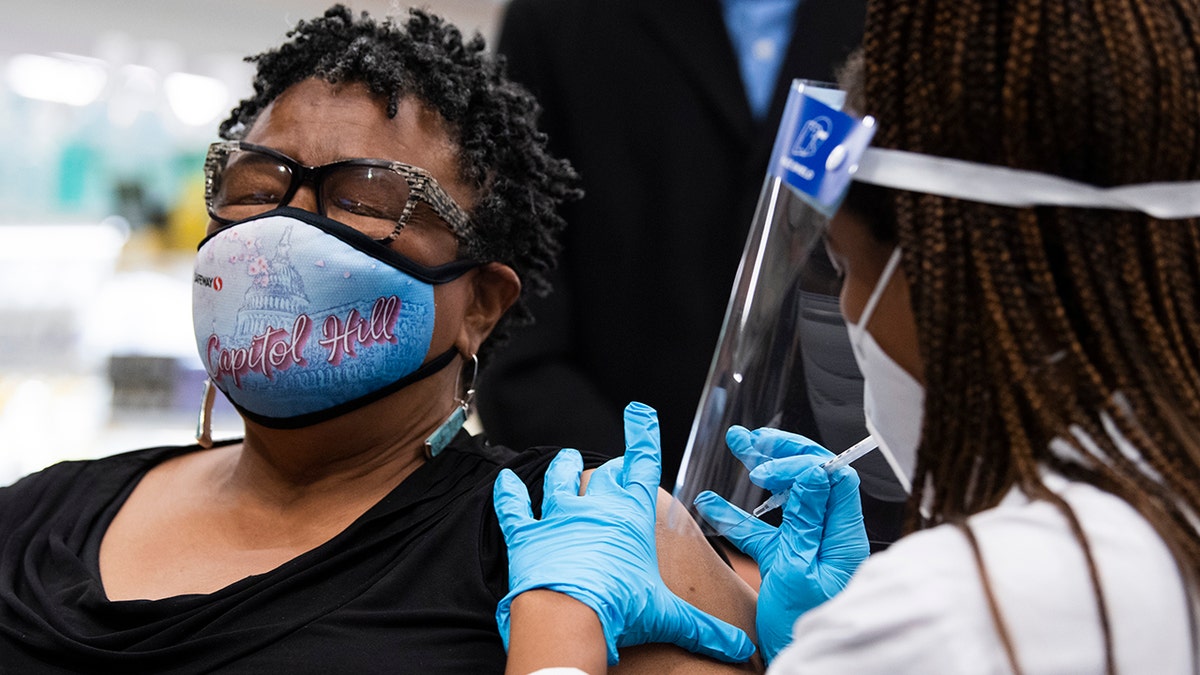
Germaine T. Leftwich, 67, receives a Pfizer covid-19 vaccine booster shot from Dr. Tiffany Taliaferro at the Safeway on Capitol Hill in Washington, D.C., on Monday, October 4, 2021. ( Tom Williams/CQ-Roll Call, Inc via Getty Images)
BIDEN HOLIDAY PLANS NOT CHANGING DUE TO COVID-19: PSAKI
A kid-sized version of Pfizer’s vaccine already is available for 5- to 11-year-olds, one that’s a third of the dose given to everyone else 12 and older.
No safety concerns have been spotted in the study, the companies said.
Pfizer vaccine research chief Kathrin Jansen cited other data showing a booster shot for people 16 and older restores strong protection, a jump in immunity that scientists hope also will help fend off the new omicron variant.

This image provided by Pfizer shows its COVID-19 pill. Drugmaker Pfizer said Tuesday, Nov. 16, 2021, it is submitting its experimental pill for U.S. authorization, setting the stage for a likely launch in coming weeks. (Pfizer via AP) (Pfizer via AP)
CLICK HERE TO GET THE FOX NEWS APP
The companies also are preparing to test a booster for 5- to 11-year-olds, who are just now getting their two-dose vaccinations. And they are testing different dose options for teen boosters.
Jansen said if the additional pediatric testing is successful, "we would have a consistent three-dose vaccine approach for all ages."
Associated Press contributed to this report





















