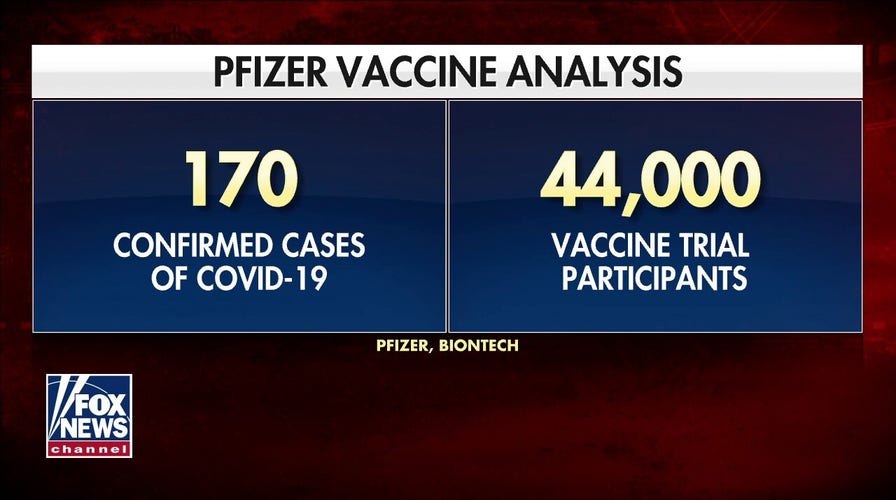
Pfizer will apply for FDA approval after announcing vaccine’s 95% percent effectiveness
Fox News medical contributor Dr. Marc Siegel with Pfizer vaccine analysis and his take on when emergency authorization will likely take place.
Pfizer and its partner BioNTech plan filed for FDA emergency approval for its coronavirus vaccine on Friday.
The two companies confirmed their intention to file on Friday in a statement released hours earlier.
Pfizer and BioNTech are currently in Phase 3 trials on the vaccine as is fellow pharmaceutical giant, Moderna.
In the press release, the companies said that their vaccine would be ready to ship "within hours" of approval.
Follow below for updates on the coronavirus. Mobile users click here.