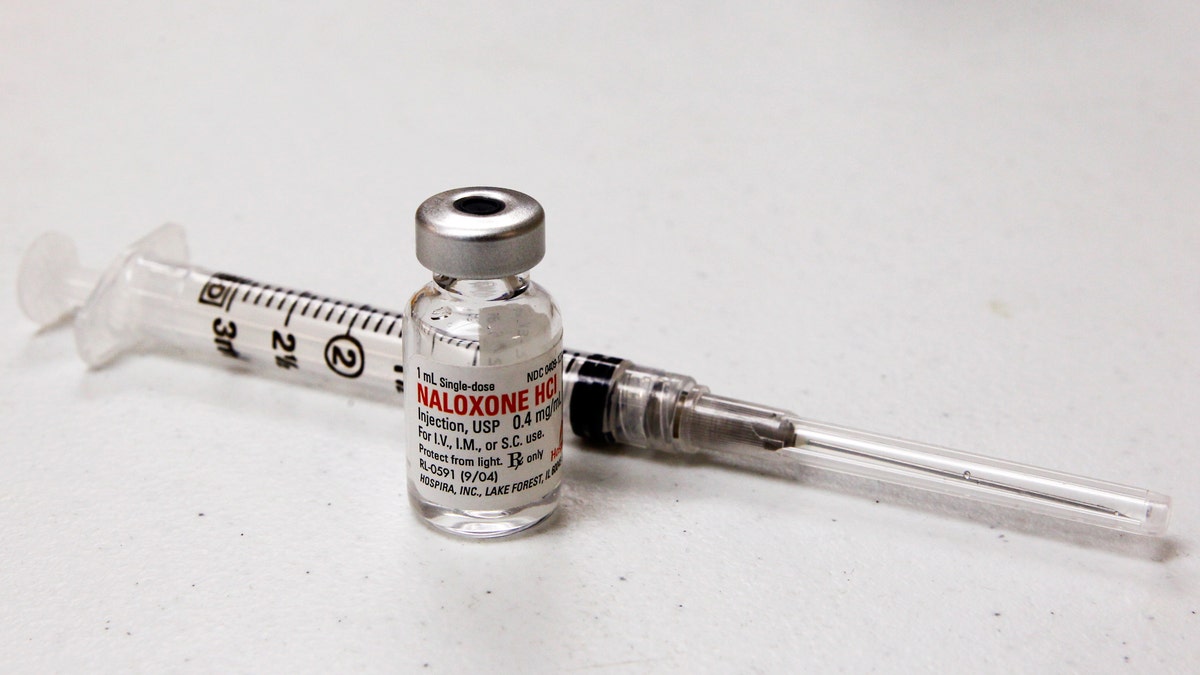
A vial of Naloxone and syringe are pictured at a Naloxone training class taught by Jennifer Stepp and her daughter Audrey for adults and children to learn how to save lives by injecting Naloxone into people suffering opioid overdoses at the Hillview Community Center in Louisville, Kentucky, November 21, 2015. REUTERS/John Sommers II - RTX1VIP5
Amphastar Pharmaceuticals Inc said on Tuesday that the U.S. Food and Drug Administration had rejected its application to market an intranasal version of the drug naloxone, which is designed to stop or reverse the effects of an opioid overdose.
The FDA's complete response letter identifies issues including user human factors study and device evaluation, the company said.
Amphastar already sells naloxone in pre-filled syringes.








































