Fox News Flash top headlines for June 15
Fox News Flash top headlines are here. Check out what's clicking on Foxnews.com.
A Food and Drug Administration outside advisory panel is poised on Wednesday to approve COVID-19 vaccines for children as young as six months old.
The advisory panel will meet for the second time on the issue to determine whether the Pfizer and Moderna vaccines are safe for the youngest Americans. The FDA has already supplied hundreds of pages of trial data it claims shows the vaccines are safe for the age group.
The FDA could move forward with an emergency authorization as early as Friday, depending on the panel's recommendation.
Pfizer's proposed regimen calls for children to receive three doses of the vaccine at one-tenth the strength of adult shots, while Moderna calls for two doses at one-fourth the strength of adult shots.
NOVAVAX COVID-19 VACCINE RECOMMENDED FOR APPROVAL BY FDA ADVISORY COMMITTEE
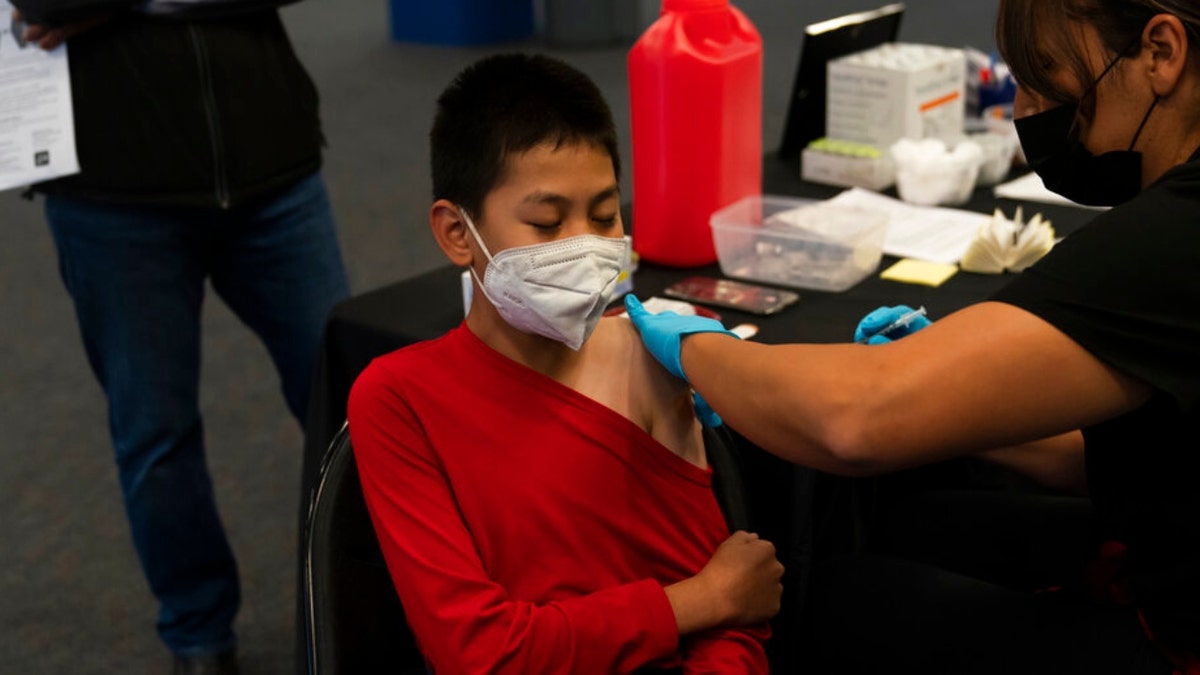
FILE - A youngster receives the Pfizer COVID-19 vaccine at a pediatric vaccine clinic for children ages 5 to 11 set up at Willard Intermediate School in Santa Ana, Calif., Tuesday, Nov. 9, 2021. (AP Photo/Jae C. Hong, File)
Pfizer's trials found that the vaccine was only effective in preventing COVID-19 infection 28% of the time in children aged six months to four.
Moderna's trials found that their regimen successfully prevented infection 51% of the time in kids aged six months to two years old, falling to 37 percent when including those up to five years old, according to the New York Times.
Children under five years old are the only age group in the U.S. that has yet to be approved for the vaccine. The CDC states that just 442 children under five years old have suffered deaths "involving coronavirus disease" since the pandemic began.
While the panel has yet to release its recommendation, President Biden's administration has already ordered millions of doses in anticipation of approval.
CLICK HERE TO GET THE FOX NEWS APP
A separate CDC advisory panel will also meet Friday and Saturday to discuss the safety of vaccines for the age group.
The CDC began recommending booster shots for children ages 5-11 in mid-May. The spread of COVID-19 variants makes it likely that the under-five age group will also receive recommendations for booster shots.










































