Fox News Flash top headlines for February 16
Fox News Flash top headlines are here. Check out what's clicking on Foxnews.com.
Frostbite may have met its match.
The U.S. Food and Drug Administration (FDA) has approved the first medication to treat severe frostbite, the agency announced this week.
The drug, iloprost (brand name Aurlumyn) is intended to reduce the risk of finger and toe amputations due to dangerously cold extremities.
NEED A 'WINTER RESET'? EXPERTS SHARE BENEFITS OF SLOWING DOWN DURING COLDER MONTHS
Iloprost was originally approved for treatment of pulmonary arterial hypertension, a condition in which high blood pressure affects arteries in the lungs and heart.
"This approval provides patients with the first-ever treatment option for severe frostbite," said Norman Stockbridge, M.D., Ph.D., director of the Division of Cardiology and Nephrology in the FDA’s Center for Drug Evaluation and Research, in a press release.

Frostbite occurs when cold temperatures cause parts of the body to freeze, primarily the fingers, toes, nose, cheeks and chin. (iStock)
"Having this new option provides physicians with a tool that will help prevent the life-changing amputation of one’s frostbitten fingers or toes."
Frostbite occurs when cold temperatures cause parts of the body to freeze, primarily the fingers, toes, nose, cheeks and chin, according to the U.S. Centers for Disease Control and Prevention (CDC).
ASK A DOC: 'HOW SHOULD I CARE FOR MY SKIN DURING THE WINTER?'
Initial symptoms include numbness, tingling or color changes, Dr. Marc Siegel, clinical professor of medicine at NYU Langone Medical Center and a Fox News medical contributor, told Fox News Digital.
"Severe frostbite can cause white or blue skin and, later, fluid-filled blisters," said Siegel, who was not involved in the drug development.
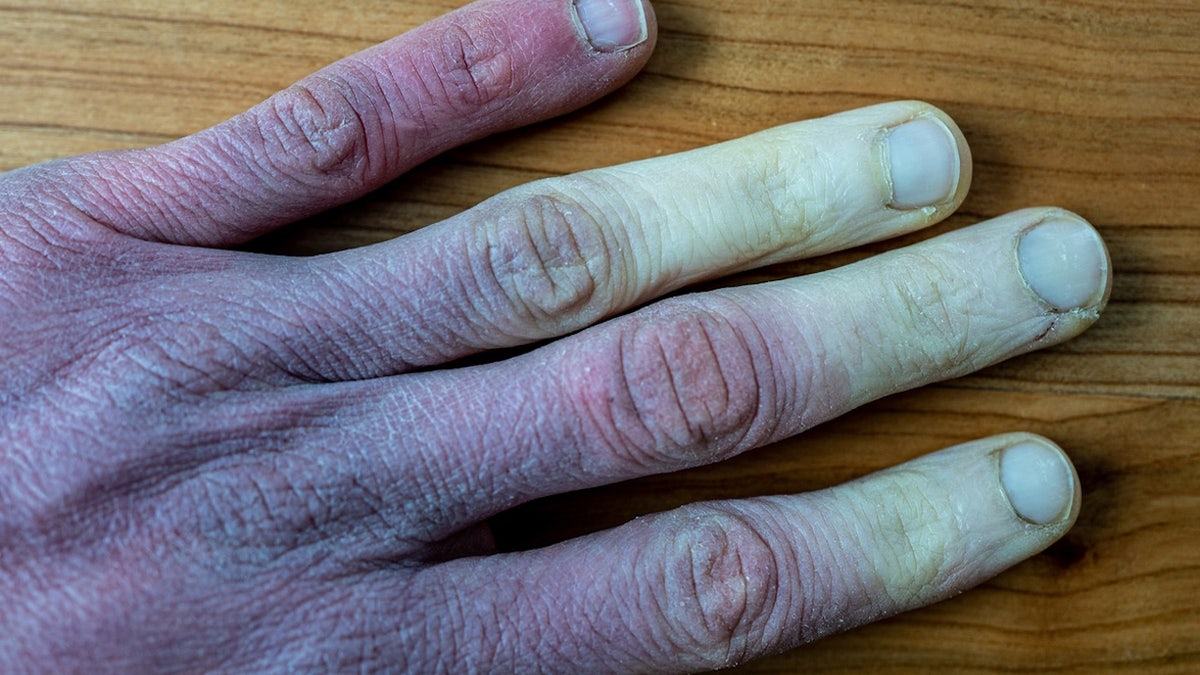
Initial symptoms of frostbite include numbness, tingling or color changes. (iStock)
Prolonged frostbite can lead to permanent damage or amputation, the CDC stated.
Aurlumyn is intended for severe frostbite cases, in which both the skin and the underlying tissue are frozen, and blood flow is stopped.
WHY YOU FEEL MORE ACHES AND PAINS IN THE COLD WEATHER — AND WHAT YOU CAN DO ABOUT IT
"Iloprost, the active ingredient in Aurlumyn, is a vasodilator, a drug that opens blood vessels and prevents blood from clotting," the FDA release stated.
The FDA’s approval follows a randomized clinical trial that included 47 adults with severe frostbite, who were divided into three groups.

Prolonged frostbite can lead to permanent damage or amputation, the CDC stated. (IStock)
One group received iloprost intravenously for six hours daily for up to eight days.
Another group received other medications that are not approved for frostbite, combined with iloprost.
A third group received other medications without iloprost.
SNOW SHOVELING SAFETY TIPS TO PREVENT INJURY AND HEART ATTACKS: 'VERY STRENUOUS ACTIVITY'
Seven days after the initial frostbite, each participant received a bone scan to determine whether fingers or toes would need amputation.
The patients who received iloprost did not require any amputation, compared to 19% of patients who received iloprost with other medications and 60% of patients who only received other medications.

Signage is seen outside the Food and Drug Administration (FDA) headquarters in White Oak, Maryland, on Aug. 29, 2020. (REUTERS/Andrew Kelly/File Photo)
"This is a very important approval," Siegel told Fox News Digital.
"Severe frostbite causes blood clotting and can lead to amputation. This new drug’s active ingredient, ilopost, is a vasodilator that preserves blood flow to the area of severe frostbite and prevented the need for amputation in all cases in a small study."
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
Aurlumyn, which was developed by Eicos Sciences Inc. in Maryland, was found to cause some side effects, the FDA noted.
Those included heart palpitations, accelerated heart rate, nausea, headache, flushing, vomiting, dizziness and low blood pressure.
CLICK HERE TO GET THE FOX NEWS APP
For that reason, Siegel noted, the drug can only be administered by a physician.
Fox News Digital reached out to Eicos Sciences Inc. and the FDA requesting additional comment.









































