Fox News Flash top headlines for August 27
Fox News Flash top headlines are here. Check out what's clicking on Foxnews.com.
The Food and Drug Administration granted Abbott Technologies emergency use authorization for its $5 COVID-19 antigen test Wednesday saying it's an "important addition to available tests."
The test -- which is said to be the size of a credit card -- is the first antigen test where results can be read directly from the testing card, according to the FDA's Wednesday announcement.
With no equipment needed, Abbott said, "The device will be an important tool to manage risk by quickly identifying infectious people so they don't spread the disease to others."
It can be administered in a doctor's office, emergency room or school with results delivered in 15 minutes.
"This means people will know if they have the virus in almost real-time," Dr. Jeff Shuren, director of the FDA’s Center for Devices and Radiological Health, said. "Due to its simpler design and the large number of tests the company anticipates making in the coming months, this new antigen test is an important advancement in our fight against the pandemic."
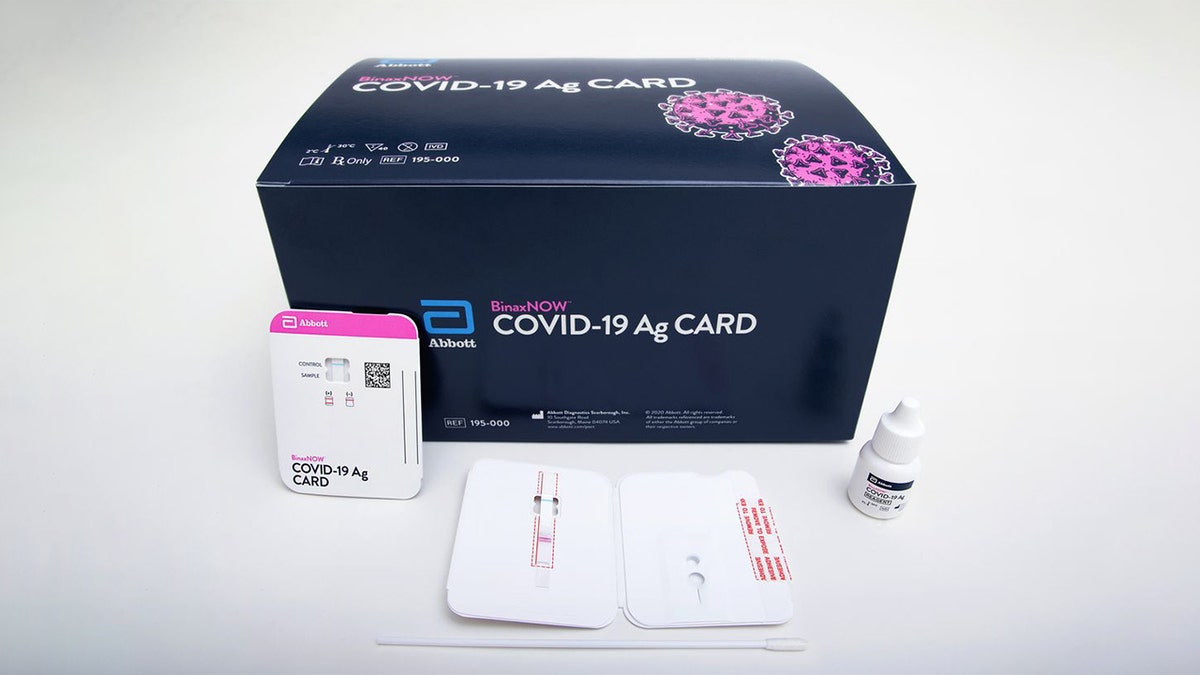
(Abbott Technologies)
The test uses similar technology that at-home pregnancy tests use.
First, a health care provider will swab a patient's nose and apply the sample to the test card. After 15 minutes, results can be read directly from the testing card with one line indicating a negative result and two lines indicating a positive result.
With rapid tests, "you get a result right away, getting infectious people off the streets and into quarantine so they don't spread the virus," said Joseph Petrosino, professor and chairman of molecular virology and microbiology at Baylor College of Medicine, whose labs have been leading efforts to provide COVID-19 testing for the college and Harris County.
Comparatively, "with lab-based tests, you get excellent sensitivity but might have to wait days or longer to get the results," added Petrosino.
FDA GREENLIGHTS NEW CORONAVIRUS ANTIGEN TEST
The test has been authorized for patients, who are believed to have COVID-19 by their health care provider, within seven days of seeing symptoms.
"Given the simple nature of this test, it is likely that these tests could be made broadly available," the FDA said.
Abbott will ship tens of millions of tests in September with plans to make up to 50 million tests available monthly in the U.S. by October.

