Dr. James Kelly shares advice on drug-resistant bacteria linked to eye drops
Kelly Vision Center founder Dr. James Kelly, M.D., discusses the outbreak of drug-resistant bacteria linked to eye drops, the investigation into the contamination and provides advice on how to decrease infection.
There have been a wave of eye drop recalls this year, with the U.S. Food and Drug Administration (FDA) recently announcing a nationwide voluntary recall of another 27 products.
On Oct. 27, the agency released a list of 26 products that consumers should not purchase and should stop using due to "the potential risk of eye infections that could result in partial vision loss or blindness."
A few days later, the FDA added one more product to the list.
MORE EYE DROPS RECALLED AFTER FDA WARNING
The products are marketed under the following brands, the agency stated in its announcement:
- CVS Health
- Leader (Cardinal Health)
- Rugby (Cardinal Health)
- Rite Aid
- Target Up & Up
- Velocity Pharma

This year has seen a wave of eye drop recalls, with the FDA announcing a nationwide voluntary recall of 27 products in the country. (iStock)
"Ophthalmic drug products pose a potential heightened risk of harm to users because drugs applied to the eyes bypass some of the body’s natural defenses," the FDA noted.
The agency called for the manufacturers to recall all lots of the specified products due to "unsanitary conditions in the manufacturing facility and positive bacterial test results from environmental sampling of critical drug production areas in the facility."
Consumers should "properly discard" the products, the FDA recommended.
RITE AID CLOSING 154 STORES IN 15 STATES: HERE'S THE LIST
"The recent uptick in recalls has been linked to potential bacterial contamination due to unsanitary and inferior quality manufacturing facilities that did not meet the required FDA ‘good practices’ in manufacturing," Dr. Ronald Benner, president of the American Optometric Association (AOA) in Missouri, told Fox News Digital.
"It’s important to note that eye drops are safe when manufactured and used properly."
On Oct. 27, the agency released a list of 26 products that consumers should not purchase and should stop using due to "the potential risk of eye infections that could result in partial vision loss or blindness." (iStock)
On Nov. 3, the FDA announced that Cardinal Health Inc. and Harvard Drug Group LLC had issued voluntary recalls for their products.
On Nov. 15, Kilitch Healthcare India Limited followed suit with its own voluntary recall.
CVS, Rite Aid and Target all said they would remove the products from their store shelves and websites, the FDA stated.
CVS PULLS POPULAR COLD MEDICINES FROM STORE SHELVES
"Patients who have signs or symptoms of an eye infection after using these products should talk to their health care provider or seek medical care immediately," the FDA said in its announcement.
Thus far, there have been no reports of adverse events related to the October recall.
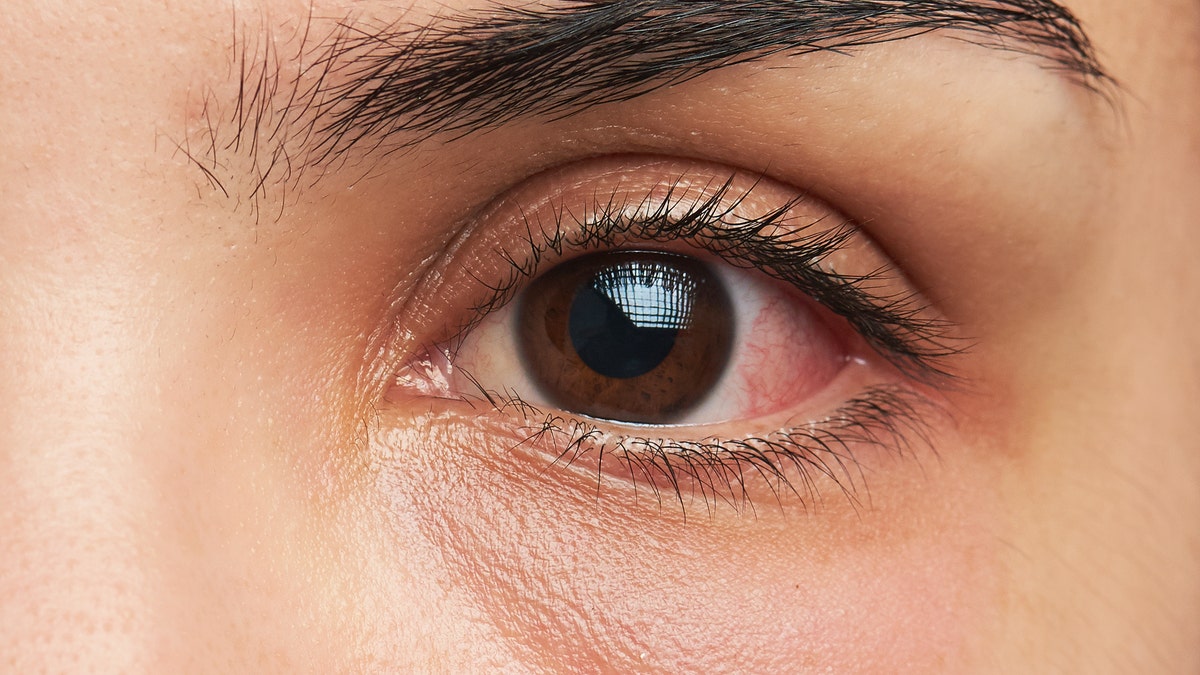
Signs of a potential eye infection symptoms may include redness of the eye or eyelid, eye pain or irritation, and watery eyes. (iStock)
Earlier in the year, however, the January 2023 recall of EzriCare or Delsam Pharma’s Artificial Tears resulted in 81 people reporting infections across 18 states, Benner noted.
"To date, 14 people have gone blind, four required enucleation and four others died," he told Fox News Digital.
The FDA can only make recommendations and does not have the authority to issue mandatory recalls — that is left up to the individual retailers and manufacturers.
What consumers should do
Using contaminated artificial tears increases the risk of eye infections that could result in blindness or death, Benner warned.
"Patients who have used these products and/or have signs or symptoms of an eye infection should seek medical care immediately," he said.
FAMILY DOLLAR RECALL OF OTC DRUGS, MEDICAL DEVICES SPANS 23 STATES
"Some eye infections can lead to further complications, especially if left untreated."
Signs of a potential eye infection symptoms may include the following, he said.
- Redness of the eye or eyelid
- Eye pain or irritation
- Watery eyes
- Feeling of something in your eye (foreign body sensation)
- Increased sensitivity to light
- Blurry vision
Even if someone has not experienced any of these symptoms but has used any of the recalled products, Benner said they should see their optometrist for a comprehensive exam. (iStock)
Even if someone has not experienced any of these symptoms but has used one of the recalled products, Benner said that person should see their optometrist for a comprehensive exam to assess the front surface of their eye.
"If you have used [one of the] products or have experienced any new and/or prolonged irritation in your eyes, visit AOA.org to book an appointment with an AOA doctor of optometry near you," he recommended.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
Anyone who owns the recalled eye drops should follow the FDA’s guidelines for throwing the products away, which may involve taking them to a drug take-back site, Benner noted.
The FDA also encourages health care professionals and patients to report adverse events or quality problems with any medicine to FDA’s MedWatch Adverse Event Reporting program.
Before consumers treat themselves with over-the-counter (OTC) eye drops, the AOA recommends consulting with their local optometrist and also checking the FDA’s website for an up-to-date list of products being recalled.

Anyone who owns the recalled eye drops should follow the FDA’s guidelines for throwing the products away, which may involve taking them to a drug take-back site. (iStock)
"When talking with your optometrist, it’s important to share any prescribed and over-the-counter medications currently in use and ask for their professional opinion," Benner told Fox News Digital. "This can also help ensure that an appropriate treatment plan is in place."
Fox News Digital reached out to all involved companies and to the FDA for additional comment.
CLICK HERE TO GET THE FOX NEWS APP
CVS Health provided the following statement to Fox News Digital regarding the product recalls: "Upon receiving notification by the FDA, we immediately stopped the sale in-store and online of all products supplied by Velocity Pharma within the CVS Health Brand Eye Products portfolio."
"Customers who purchased these products can return them to CVS Pharmacy for a full refund. We’re committed to ensuring the products we offer are safe, work as intended and satisfy customers, and are fully cooperating with the FDA on this matter."
Rite Aid provided the following statement to Fox News Digital:
"Due to safety concerns identified by the FDA, we have removed applicable Rite Aid-branded recalled products from our store shelves. Customers can return applicable recalled eye drops to Rite Aid locations for a refund."





















