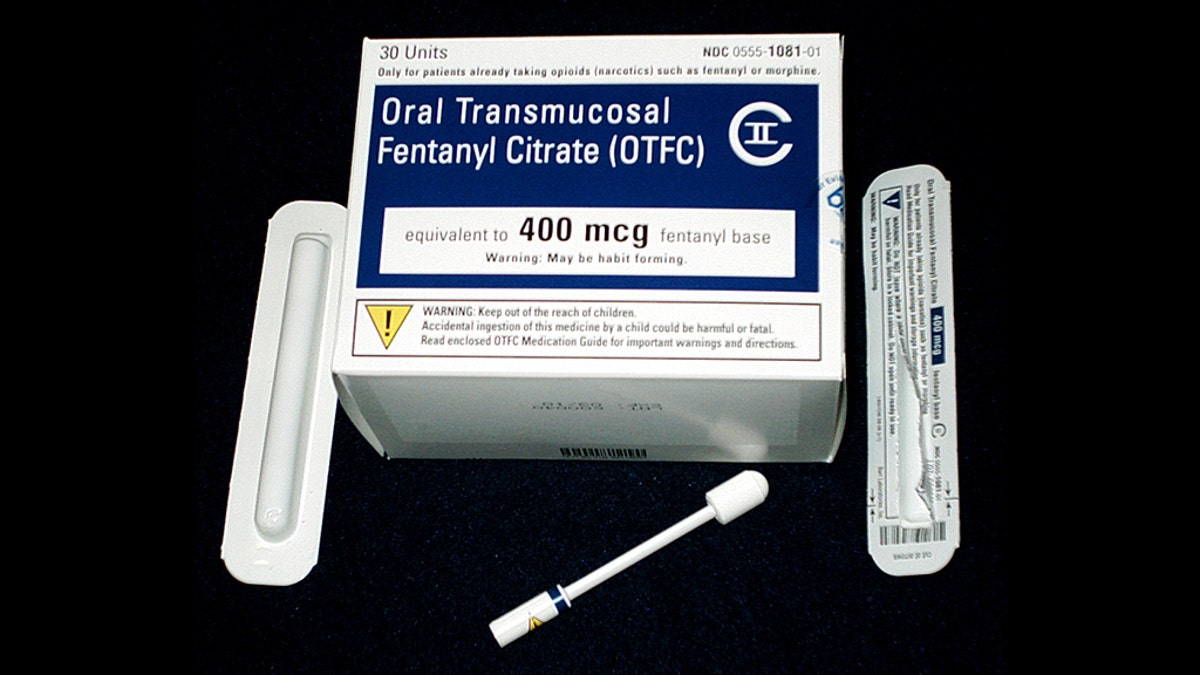
Prescription fentanyl
Prescription fentanyl, a highly potent and immediate-release drug meant for people with advanced cancer who have been on an opioid treatment, has been inappropriately prescribed to people who did not meet the criteria, says a new study by Johns Hopkins University researchers.
The researchers, using more than 4,000 pages of Federal and Drug Administration documents they obtained through the Freedom of Information Act, or FOIA, found that the drug was wrongly prescribed up to 50 percent of the time.
FDA WARNS AGAINST 'YOUNG BLOOD' TRANSFUSIONS
What’s more, they found, a federal system was set up to track these fentanyl prescriptions practices did not flag a single one of the prescribers who had been giving the drug incorrectly. The system, the study said, aimed "to reduce the risk of adverse outcomes, including misuse, abuse, addiction, and overdose, arising from use of [the drug]"
“Because of their unique risk, they’re subject to one of the most restrictive distribution systems that the FDA has,” said the study’s author, Caleb Alexander, a Johns Hopkins University professor of medicine, to Fox News. “Prescribers, pharmacists and patients all have to be especially certified to access this product. It’s a really big deal.”
“The risks, when used unsafely, are enormous. These products can kill," Alexander said. "The prescription monitoring system for this drug did not function as intended. Both the FDA and manufacturers missed an opportunity to improve the safety of these products.”
HEALTH EXPERTS OFFER SOLUTIONS FOR UNINTENDED CONSEQUENCES OF OPIOID CRACKDOWN
Alexander stressed that the number of Americans who use this type of fentanyl -- which is not the one that comes in patch form but as lollipops, nasal spray or lozenges -- is a small fragment of the millions who take prescription opioids.
When correctly prescribed, this drug, known as transmuccosal immediate-release fentanyl, or TIRFs, brings relief to cancer patients with intractable pain that is so intense that even other prescription opioids do not help sufficiently, Alexander said.
Researchers found, however, that prescription fentanyl meant for people with these severe conditions were being given to people for such things as headaches, back pain and fibromyalgia.
“Doctors and patients have to be aware that they should be used for people who already are on the around-the-clock opioids,” Alexander said, “And many receiving it were not.”
Alexander wanted to stress that TIRFs are different from the illicitly manufactured fentanyl that, along with heroin, is fueling the opioid overdose epidemic.
CLICK HERE TO GET THE FOX NEWS APP
Dr. Stefan Kertesz, an addiction specialist and professor at the University of Alabama at Birmingham School of Medicine, said the Johns Hopkins study, with which he was not involved, “makes very clear that basic rules of the road for dealing with a highly specialized opioid were not met and it highlights shortfalls in American pain care and our prescribing culture.”
“Solid reporting suggests that inappropriate marketing contributed to irresponsible prescribing,” Kertesz said. “There is no reason to prescribe a highly specialized and potent opioid like this outside of very specialized situations.”








































