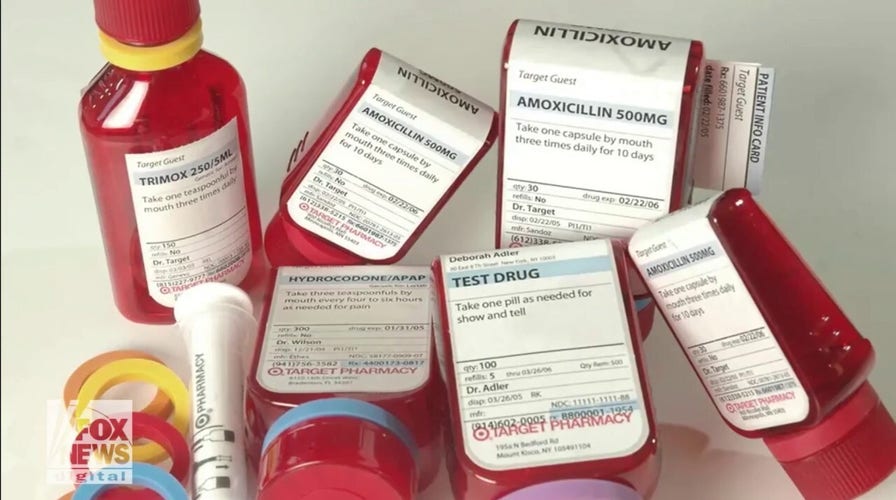
Deborah Adler built a better RX bottle – here’s how a grad-school student's project made our medicine cabinets safer
Born into a family of caregivers in New York, Deborah Adler turned her concern for her grandmother into a business move that improved the way Americans take prescription medicine.
The anxiety-reducing drug, Clonazepam, has been recalled after a potentially "life-threatening" label mix-up, the Food and Drug Administration (FDA) said.
According to a release from the federal agency, Endo Inc. announced a voluntary recall of 16 lots of Clonazepam Orally Disintegrating Tablets.
The pharmaceutical company said the immediate recall came after it was discovered that 16 lots of the anxiety drug were mislabeled with the incorrect strength and National Drug Code (NDC) on them. The company said the labeling error was made by a third-party packager.
As a result, children and adults prescribed Clonazepam could face "life-threatening" side effects, the FDA warned.

The recalled products were distributed to pharmacies nationwide in cartons containing 60 tablets packed into 10 blister strips of six tablets. (FDA)
The mislabeling of the drug could result in "significant sedation, confusion, dizziness, diminished reflexes, ataxia, and hypotonia," the FDA said.
"There is reasonable probability for significant, possibly life-threatening, respiratory depression especially for patients with concomitant pulmonary disease, patients who have prescribed dosing near maximal dosing, and patients also taking other medications that could cause additional respiratory depression," the FDA said.
Endo Inc. noted that, as of Nov. 21, there have not been any reports of adverse effects from the product recall.

The company said the labeling error was made by a third-party packager. (FDA)
The following table, provided by the FDA, details the lots being added to the voluntary recall, including lot product description and NDC number:
| Potential Product Description / NDC Number | Lot # |
| Clonazepam ODT, USP (C-IV) 2mg / 49884-310-02 | 550176501 |
| 550176601 | |
| Clonazepam ODT, USP (C-IV) 0.125mg / 49884-306-02 | 550174101 |
| Clonazepam ODT, USP (C-IV) 0.25mg / 49884-307-02 | 550142801 |
| 550142901 | |
| 550143001 | |
| 550143101 | |
| 550143201 | |
| 550143301 | |
| 550143401 | |
| 550147201 | |
| 550147401 | |
| Clonazepam ODT, USP (C-IV) 1mg / 49884-309-02 | 550145201 |
| 550175901 | |
| 550176001 | |
| 550176201 |
Individuals with unused prescribed tablet cartons of Clonazepam Orally Disintegrating tablets bearing the above lot numbers have been advised to discontinue use of the product.
BREAST CANCER VACCINE UPDATE FROM CLEVELAND CLINIC: ‘A NEW ERA’
In the event that a patient inadvertently took an incorrect dose rather than the intended dose, they are advised to consult a physician, the FDA said.
Consumers with questions about the recall can contact Inmar Inc., the company handling the recalls, by telephone at 855-589- 1869 or by email at rxrecalls@inmar.com.

Signage is seen outside the Food and Drug Administration (FDA) headquarters in White Oak, Maryland. (REUTERS/Andrew Kelly/File Photo)
Clonazepam tablets treat seizures and can also be used to treat panic disorder, according to the Cleveland Clinic.
CLICK HERE TO GET THE FOX NEWS APP
"It works by helping your nervous system calm down," the Cleveland Clinic said. "It belongs to a group of medications called benzodiazepines."