(iStock)
A ruling last week on the morning-after pill, as well as government recommendations on new forms of birth control, could have long-lasting effects on women's perceptions of its safety, health experts say.
Last Wednesday, the health secretary for the first time overruled government scientists, refusing to make the morning--after pill available to users of all ages without a prescription.
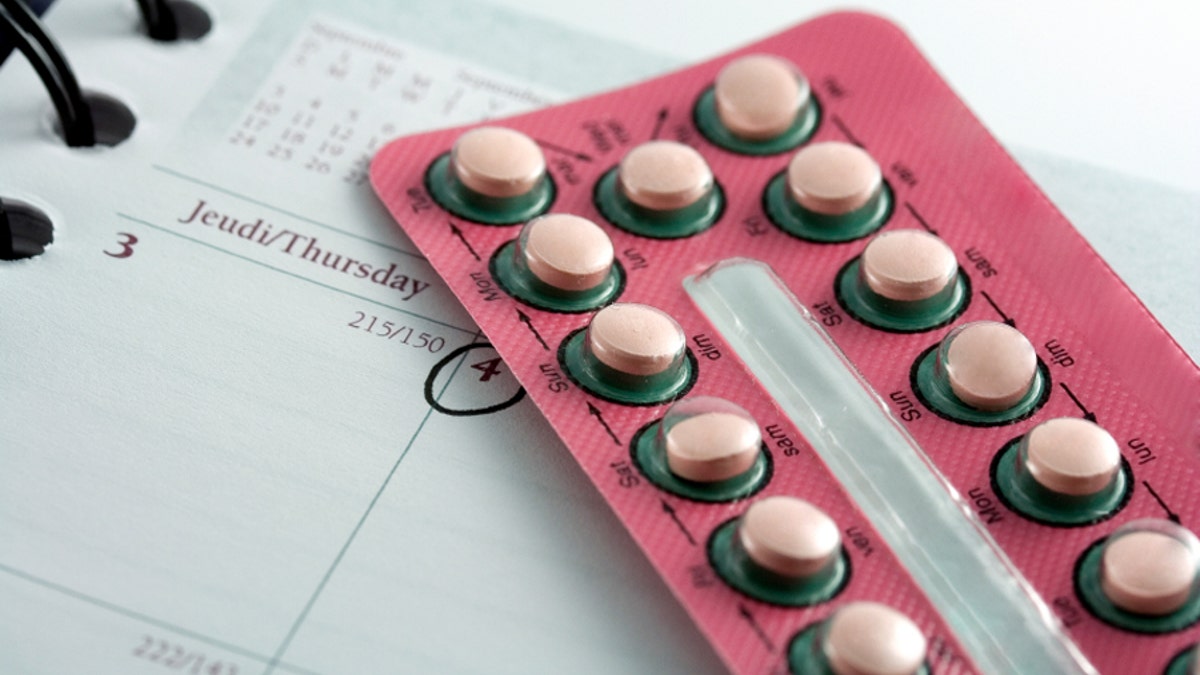
In the days that followed, advisers to the Food and Drug Administration recommended revised labels on the best-selling class of birth control pills, as well as for a contraceptive patch, to better convey their higher risk of blood clots.
Some women's advocacy groups worried the negative attention on the blood clot risk of a new generation of pills that contain drospirenone -- including Bayer AG's popular Yaz and Yasmin -- would create concerns about birth control in general.
An FDA study estimated that 10 in 10,000 women taking the drospirenone-containing drugs would get a blood clot per year, compared with about six in 10,000 women taking older contraceptives.
A clot in the blood vessels can prove fatal if it breaks loose and travels to the lungs, heart or brain.
"If you've seen on TV somebody crying that their daughter died taking birth control pills, and you're a mom, you may not remember the (particular) birth control pill," said Diana Zuckerman, president of the National Research Center for Women & Families. "You'll just say you can't be on it to your daughter."
While Zuckerman and others called for the drospirenone-containing pills to be removed from the market because women have safer options available, Planned Parenthood said the pills should remain on sale.
Dr. Vanessa Cullins, vice president for medical affairs at Planned Parenthood Federation of America, said that when the issue of blood clots first arose in 1995 with a new generation of pills in Britain, many women abandoned birth control altogether, leading to higher rates of unwanted pregnancies or abortions.
"What we know is that a focus by the media on meetings such as this carries the risk of creating panic among everyday women and also healthcare providers who may not be knowledgeable about why the meetings are being held and the data that is conveyed during the actual meetings," Cullins told Reuters.
MORE CONVERSATIONS
The better outcome of a new focus on birth control may be to encourage women to question their doctors more thoroughly about the best kind of contraception for them, others said.
Dr. Valerie Montgomery Rice chaired the FDA panel on Friday, which recommended that the label for Johnson and Johnson's Ortho Evra birth control patch be simplified to better explain the risk of blood clots.
The attention "is going to raise concerns but I hope that it does more than just that, (that it) raises awareness and pushes women to have more thorough conversations with their providers," said Rice, dean and executive vice president of the Morehouse School of Medicine.
A study from the non-profit Guttmacher Institute that drew on government data showed 47 percent of sexually-active teenagers were using hormonal contraceptives in 2008-10, compared to 37 percent in 2006-08.
Joyce Brink, 80, said she would still advise her daughters, both mothers in their 50s, and her 12- and 13-year-old granddaughters to take the pill if advised by their doctors.
"There are all sorts of side effects of almost any medication," said Brink, who was visiting Washington on Friday from Nevada. "Women have to protect themselves."
But for some, the week's events simply reinforce just how far what happens in Washington is removed from the daily lives of women.
Dr. Michele Curtis, an obstetrician and gynecologist at the University of Texas-Houston Medical School, said this week's moves would not change her opinion on birth control or what she prescribes to her patients.
"I don't think any of the stories this week should change somebody's opinion of the risk that combined contraceptives pose," she said, pointing out that the blood clot risks of the new generation of pills was known before the FDA's meetings.
"You can label it differently to try to protect yourself from the attorneys. But I'm not sure that's going to make a difference to physicians.
"All I want to know is whether it'll do more harm than good," Curtis said.
Some advocates said the morning-after pill decision by Health Secretary Kathleen Sebelius would dominate headlines, erasing any broader message from the FDA meetings about the safety of all birth control pills.
Sebelius' decision also sounded a contrary note to her other policies, including a decision in August to mandate insurance coverage of birth control, including the morning-after pill.
They said Sebelius's ruling to limit the morning-after pill to women 17 and older goes against President Barack Obama's pledge to reassert the power of science in his administration's decisions. Obama said a day later that he supported the decision.
Critics say the morning-after-pill -- meant to be taken up to 72 hours after unprotected sexual intercourse to prevent a pregnancy -- could lead to promiscuity, sexual abuse and fewer visits to the doctor if it is readily available for purchase.
"The scientific review that happened at the FDA is completely overshadowed in the larger message that birth control gets politicized all the time," said Kirsten Moore, president and CEO of the Reproductive Health Technologies Project, an advocacy group in favor of giving women more contraceptive options.
"The majority of the news that's going to break through is about contraception being political, rather than whether it's safe or not."
