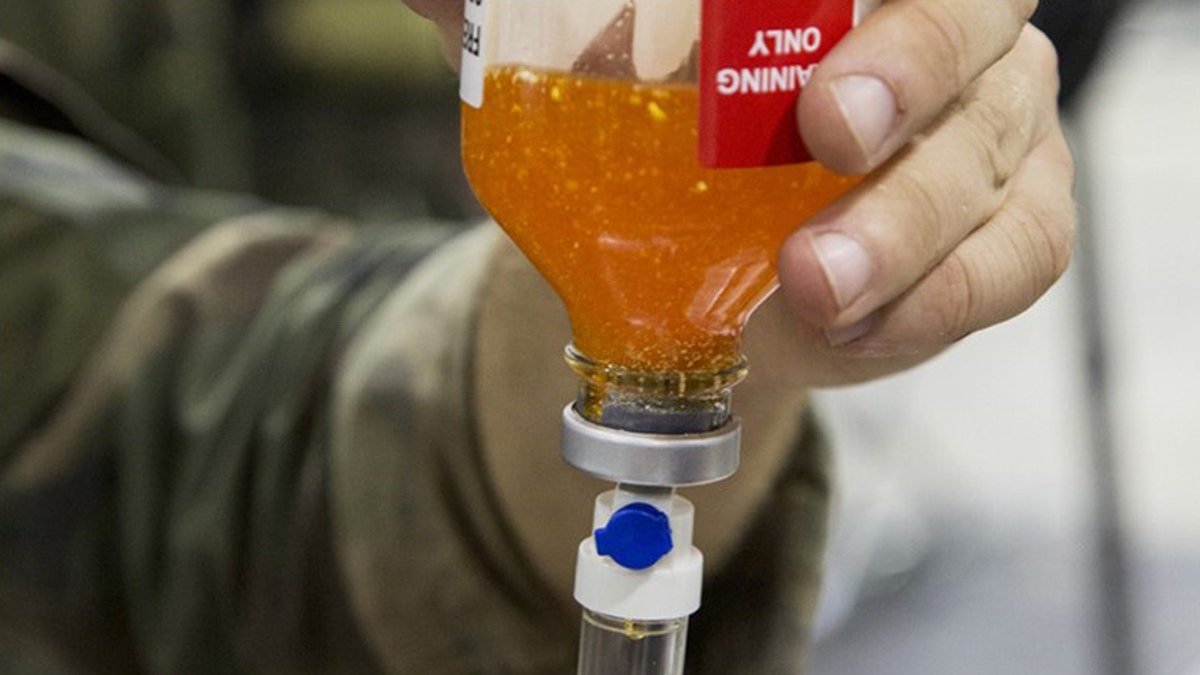
CAMP SHELBY JOINT TRAINING CENTER, Miss. - Critical Skills Operators with U.S. Marine Corps Forces, Special Operations Command simulate administering freeze-dried plasma to a role-playing casualty during a Raven exercise at Camp Shelby Joint Force Training Center, Miss., May 1, 2017. (Photo By: Sgt. Salvador R. Moreno)
Starting next week, all U.S. Navy corpsmen serving with the Marine Corps Forces Special Operations Command (MARSOC) overseas will be equipped with freeze-dried blood plasma. The decision to have all units carry the lifesaving blood product comes after it was used earlier this year to save the life of a wounded military member from an allied nation.
But the freeze-dried plasma (FDP) used in that rescue was provided to the Marines by an allied country, as will all the FDP the Marines will be carrying in the foreseeable future. That’s because, even though America’s European allies have been using FDP in the field for more than 20 years, its manufacture in the United States still hasn’t been approved by the Food and Drug Administration.
Plasma — a mixture of water, sugar, fat, protein, salts and blood components including red blood cells, white blood cells and platelets — contains proteins that help to deliver nutrients and even medicines while preventing blood vessels from collapsing and clogging. It also contains clotting factors that stop bleeding, “making it an important blood product to have on the battlefield,” according to the Armed Services Blood Programs.
US NAVY WILL USE XBOX CONTROLLERS TO OPERATE SUBMARINE PERISCOPES
Freeze-dried plasma, like other freeze-dried products, is lightweight and requires no refrigeration. Just add water and mix.
“It is stable in the field, unlike whole blood or if we were to do fresh plasma or frozen plasma, so our guys can carry it with them in their resuscitative packs,” said MARSOCC force surgeon U.S. Navy Capt. Necia Williams, in a statement.
And unlike much heavier fresh frozen plasma, which is stored at about 4 degrees below zero Fahrenheit and requires a 45-minute thawing process, FDP reconstitutes in roughly six minutes. Patients’ vital signs begin to improve minutes later, and the time saved gives the military more time to transfer wounded patients to a hospital where they can receive full medical care, said U.S. Navy Lt. Aaron Conway, a Marine Raider Regiment surgeon, in a statement obtained by the U.S. Naval Institute.
DSEI 2017: INSIDE THE WORLD’S LARGEST MILITARY TECH SHOW
“They can quickly reconstitute it, infuse it to somebody and it buys time that is so critical,” said Williams.
The process for making FDP has been around since World War II, when it involved pooling plasma from up to 1,000 donors, which increased the risk of spreading blood-borne infections like Hepatitis B.
But donors undergo more rigorous testing for diseases than they did 75 years ago, and FDP is widely used by many American allies. In 2010, Adm. William McRaven, then commander of Special Operations Command, learned that Allied forces were successfully using FDP in Afghanistan and Iraq, and he sought to make it available to U.S. forces. A special arrangement was made to allow deployed forces to use French-made FDP while the military worked with U.S. pharmaceutical firms to create an FDA approved product.
USS GERALD R. FORD IN PICTURES
The use of FDP has been allowed within U.S. Special Operations Command, with MARSOC the second service component within U.S. Special Operations Command to receive approval for its use.
Freeze-dried plasma now is expected to receive FDA approval by 2020. In the meantime, American troops will continue to rely on their European allies for their supplies.