Nguyen Thi Ngoc, 13, practices her writing at home in Ho Chi Minh City, Viet Nam in 1999. (Reuters)
The same mechanism that causes children with a rare genetic disease called progeria to age at seven times the normal rate may play a role in normal aging as well, government researchers said.
The study led by Dr. Francis Collins, director of the National Institutes of Health, suggests aging may not simply be a gradual wearing out of cells.
Instead, it may be an active biological mechanism -- one that might be tinkered with to address age-related diseases.
"I think a lot of people in the past have assumed that the aging of cells and of individuals was just a matter of everything running down," Collins told Reuters in a telephone interview.
"What we are learning at the cellular level ... is that is not right," said Collins, whose study appears in the Journal of Clinical Investigation.
Scientists for several years have been working to understand the key biological processes that trigger aging in hopes of discovering new drugs that could delay or prevent age-related diseases such as cancer, heart disease and Alzheimer's disease.
Much of that has been focused on studying protective caps on the tips of chromosomes called telomeres, which Collins likens to "the aglets on shoelaces that keep the laces from them from getting ratty."
When telomeres become too short and frayed through cell division, the cell eventually dies. But it has not been entirely clear how this comes about.
Based on the study by Collins and colleagues at the National Human Genome Research Institute, it now appears that the same toxic protein that drives the premature aging disorder progeria plays a key role in normal cell aging, Collins said.
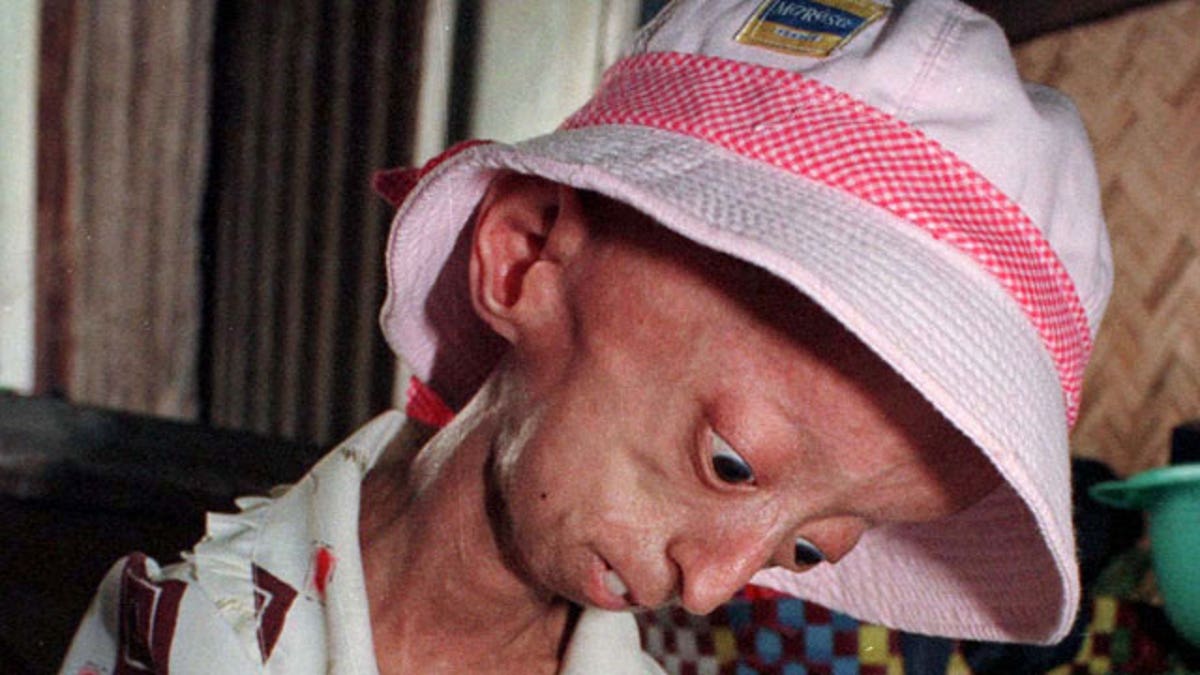
Formally known as known as Hutchinson-Gilford Progeria Syndrome, progeria is an extremely rare disease in which children experience symptoms normally linked with old age -- hair loss, wrinkled skin, clogged arteries and arthritis.
Children with the disease often die by the age of 13.
Insight Into Aging
In a 2003 study, Collins and colleagues found the disease is caused by mutations in a gene called LMNA that makes the toxic protein progerin.
"What this paper does is show the process that is happening in those children is clearly an important process in normal aging," Collins said.
"That same toxic protein that we call progerin, which is made in large quantities in those kids, is also made in your cells and mine" as the cells start to die.
"And it shows the telomeres and progerin are connected," Collins said. "They have been on parallel scientific pathways and we have now found they are actually linked together."
He said when telomeres become too short and frayed, this triggers the production of progerin, signaling to the body that the cell is at the end of its useful life.
Collins says the study shows that instead of being a passive wearing out of cells, aging is an active biological mechanism that is programed into cells.
And understanding this mechanism could lead to new kinds of treatments. Already, a study is underway in children with progeria to see if researchers could block the excess production of progerin.
Collins says more work is needed to understand the biological mechanism of aging.
"We clearly don't have the whole picture," he said.
But understanding the aging process could lead to new ways to slow normal aging, and it underscores the need to continue funding research on rare diseases.
"It is often the insights that come from the rare diseases that teach us something about more common ones," he said.
